Application of Chiral Metal Supramolecular Compounds in the Preparation of Anti-tumor Stem Cell Drugs
A technology of supramolecular compounds and stem cells, which is applied in the application field of chiral metal supramolecular compounds in the preparation of anti-tumor stem cell drugs, can solve the problem of poor selectivity of telomeric G-four-strand DNA, and achieve strong selectivity and short time , the effect of inhibiting the properties of tumor stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
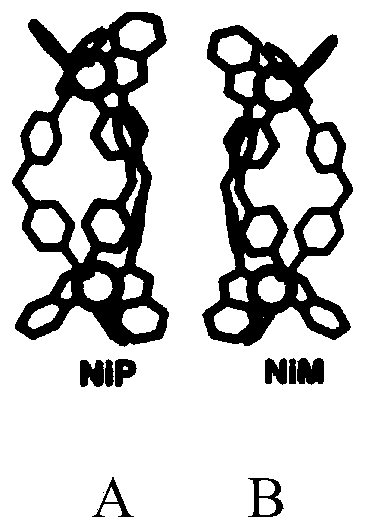
[0028] The synthesis of embodiment 1Ni-P and Ni-M
[0029] Mix 2-pyridinecarbaldehyde and 4,4'-methylenediphenylamine in an ethanol solvent at a molar ratio of 2:1, react at room temperature for 2 hours, then filter with suction to obtain a light yellow product, and dry it in vacuum to obtain a compound with the structural formula I. body; the ligand and metal chloride NiCl 2 Mix according to the molar ratio of 3:2, heat and reflux in methanol for 6h, concentrate the reaction solution, and perform chiral cellulose column separation to obtain Ni-P and Ni-M. The compound structural formula of Ni-P and Ni-M is as follows figure 1 Shown in panels A and B.
Embodiment 2
[0030] Example 2 Enrichment culture and identification of breast cancer stem cells
[0031] MDA-MB-231 and MCF-7 cells were inoculated into DMEM / F12 medium at a density of 5000 cells / ml, to which 20ng / ml basic fibroblast growth factor, 20ng / ml human recombinant epithelial growth factor, 4μg / ml heparin, 1% streptomycin and penicillin, and culture in a 6-well ultra-low adhesion culture plate, after inoculation, place the culture plate at 37°C, 5% CO 2 , in a humid incubator, and after 7 days, the formed microspheres were digested into single cells with 0.25% trypsin, and then cultured for the second time at a cell density of 5000 cells / ml. After 7 days, the microspheres were collected, and the same digested into single cells.
[0032] Will 1×10 6 Each cell was dispersed in 100 μl of PBS buffer, PE-labeled CD24 antibody and APC-labeled CD44 antibody were added to it, incubated at room temperature for 30 min, then washed twice with PBS, resuspended in 500 μl PBS, and finally an...
Embodiment 3
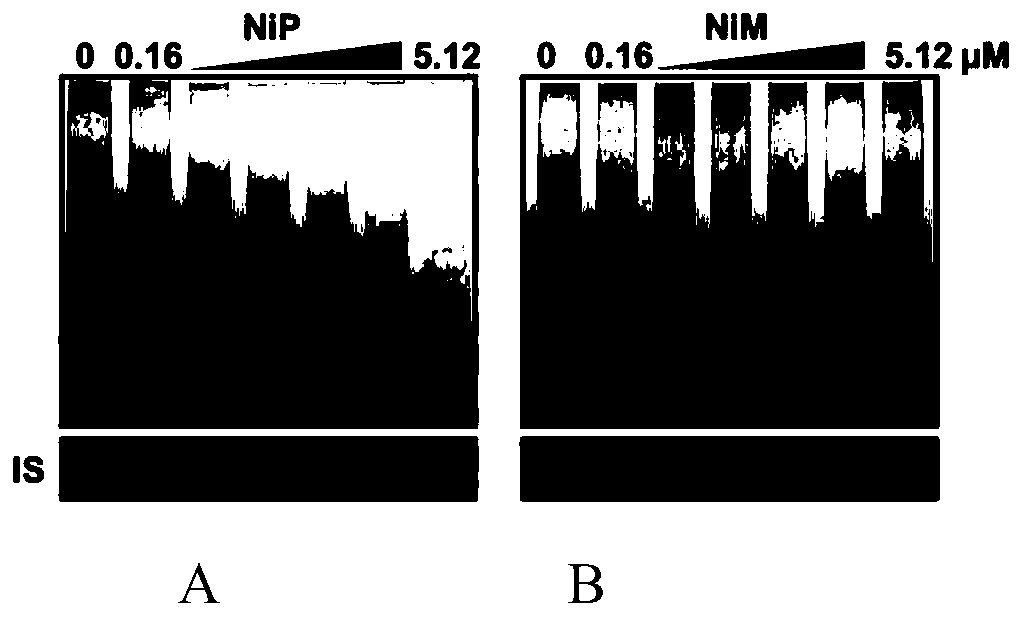
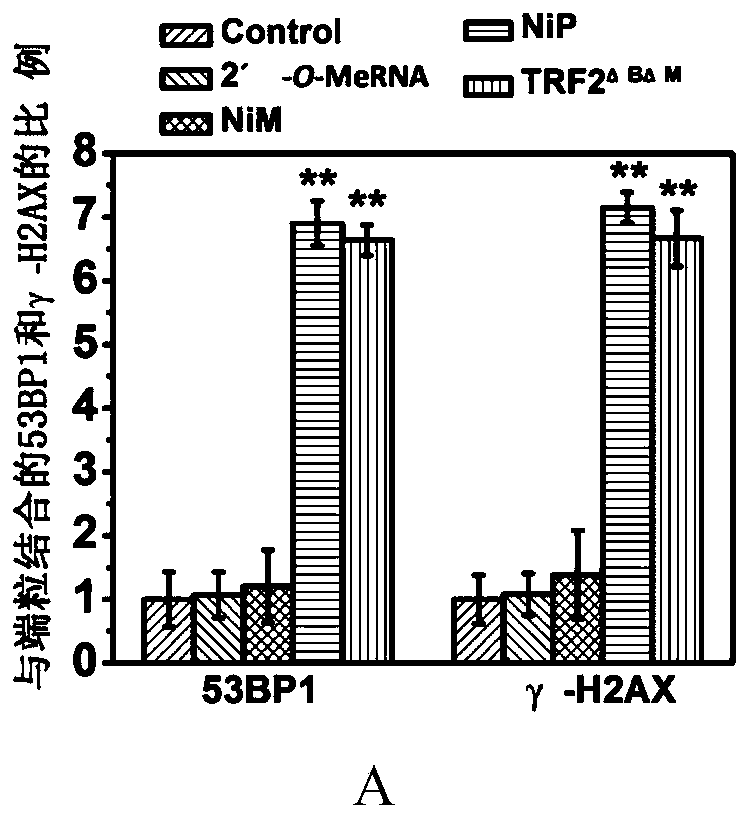
[0034] Example 3 Effects of Ni-P and Ni-M on Telomerase Activity in Tumor Stem Cells
[0035] 5,000 breast cancer stem cells were inoculated into 6-well ultra-low adhesion culture plates, and Ni-P and Ni-M were added at the same time. The concentration gradients of Ni-P and Ni-M were: 0.16, 0.32, 0.64, 1.28, 2.56, 5.12μM, cells were collected after 7 days for TRAP detection.
[0036] The TRAP experiment method is as follows: collect cells by centrifugation, wash twice with pre-cooled PBS, add 100 μL CHAPS lysate (10mM Tris HCl, pH 7.5, 1mM MgCl 2 , 1mM EGTA, 0.1mM PMSF, 0.1mMmercaptoethanol, 0.5% CHAPS, 10% glycerol), incubate on ice for 30min, centrifuge at 12000rpm, 4°C for 20min, and collect the supernatant as the crude telomerase extract. Add 3 μg of telomerase extract to the RNA inactivation system, which includes 1×TRAP buffer (20mM Tris HCl pH 8.3, 1.5mM MgCl 2 , 63mM KCl, 0.005% Tween 20, 1mMEGTA, BSA 0.1mg / ml), 0.1μg TS primer, 1mM dNTP, extended at 30°C for 60min. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com