Pyraclostrobin crystal form and preparation method thereof
A technology of pyraclostrobin and crystal form, which is applied in the field of pesticide crystallization, can solve problems such as easy coalescence, low yield, and low melting point, and achieve the effects of simple operation, good repeatability, and good process yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
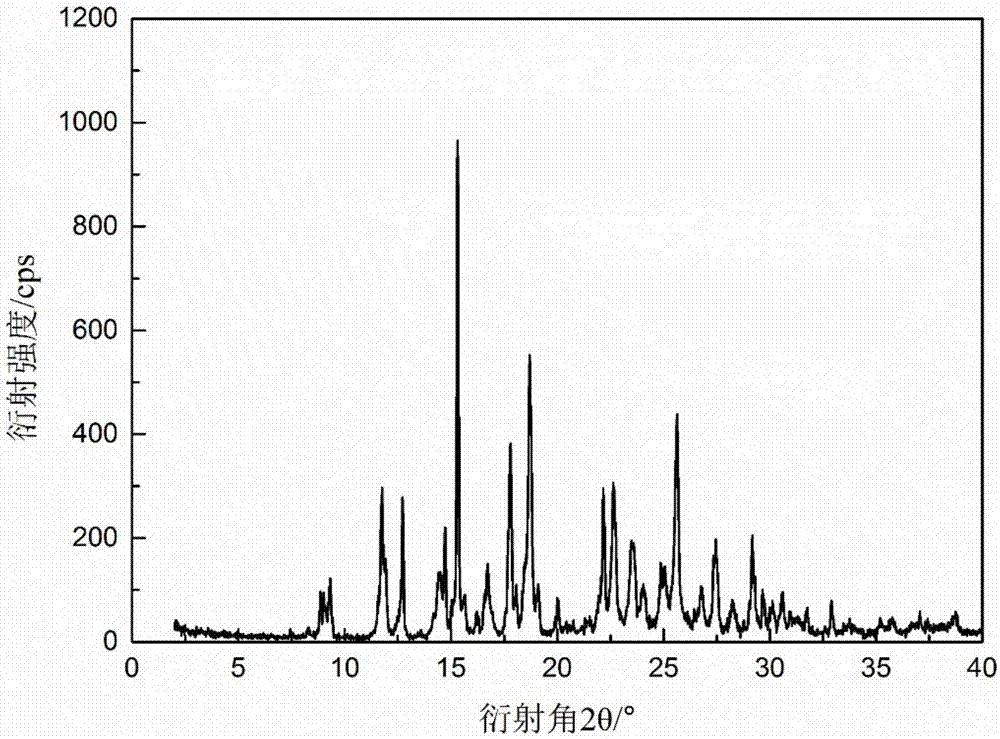
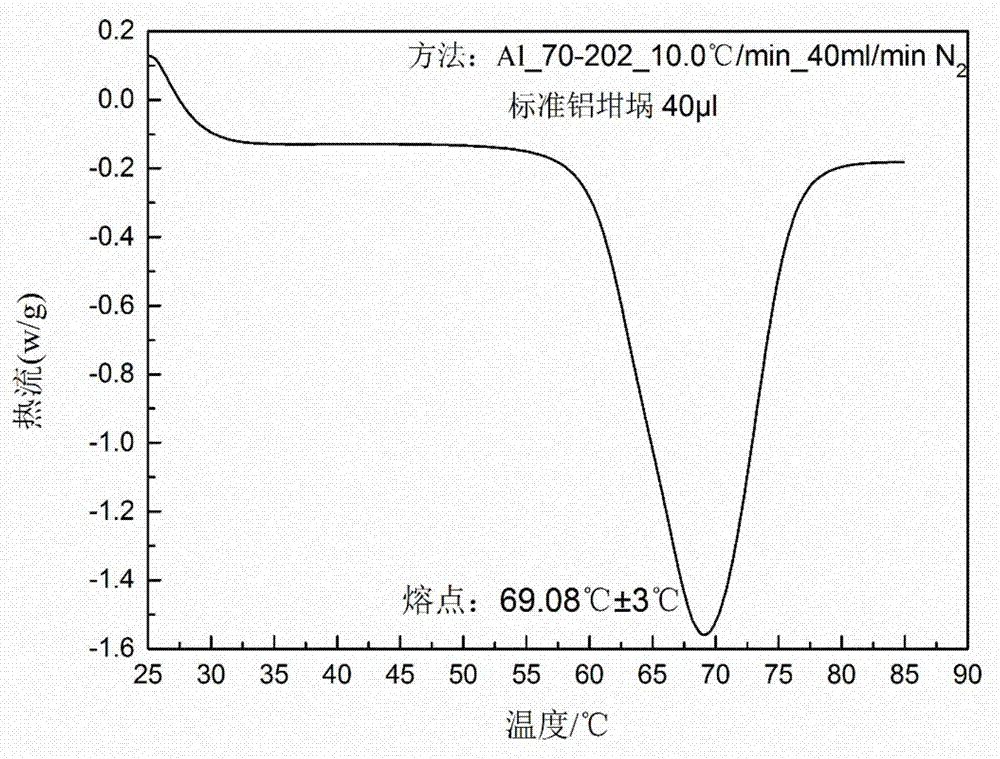
[0032] Weigh 120g of pyraclostrobin into a beaker, add 100mL of dichloromethane, stir and make it fully dissolved, evaporate the solvent at an evaporation rate of 5ml / h, and continue to distill 5ml without adding seeds after the solution reaches saturation. A total of 90 mL of solvent was evaporated at an evaporation rate of / h, filtered, washed, and the product was vacuum-dried at 35° C. for 7 hours to obtain 115 g of product with a yield of 96% and a purity of 99.9% by HPLC. The powder X-ray diffraction pattern of the product and figure 1 In agreement, the DSC spectrum is consistent with figure 2 unanimous.
Embodiment 2
[0034] Weigh 50g of pyraclostrobin into a beaker, add 60ml of dichloromethane and 40ml of cyclohexane, stir and dissolve it fully, evaporate at an evaporation rate of 30ml / h, the solution reaches saturation after evaporating for 30min, add 1g of Continue to evaporate 70ml of the solvent at an evaporation rate of 30ml / h, filter, wash, and vacuum-dry the product at 50°C for 8h to obtain 46.3g of the product with a yield of 93%. The purity was detected by HPLC 99.9%. The powder X-ray diffraction pattern of the product has characteristic peaks at 8.89, 9.05, 9.29, 11.75, 12.73, 14.72, 15.33, 17.80, 18.71, 22.16, 22.62, 25.64 degrees, and DSC shows that its melting point peak is 68.52°C.
Embodiment 3
[0036] Weigh 20g of pyraclostrobin into a beaker, add 50ml of dichloromethane and 50ml of n-hexane, stir and dissolve it fully, evaporate the solvent at an evaporation rate of 10ml / h, and when the solution reaches saturation Continue to evaporate the solvent at an evaporation rate of 10ml / h under the conditions to a total of 80mL, filter, wash, and vacuum-dry the product at 40°C for 8h to obtain 18.2g of product with a yield of 91% and a purity of 99.9% by HPLC. The powder X-ray diffraction pattern of the product has characteristic peaks at 8.87, 9.04, 9.27, 11.75, 12.72, 14.71, 15.32, 17.79, 18.69, 22.15, 22.61, 25.64 degrees, and DSC shows that its melting point peak is 69.73°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com