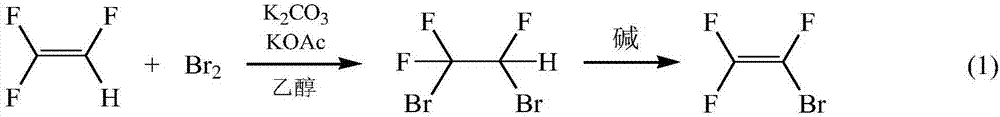
Method using phase-transfer catalysis to prepare trifluorobromoethylene
A technology of trifluoroethylene bromide and phase transfer catalyst, which is applied in the fields of dehydrohalogenation preparation, organic chemistry, etc., can solve the problems of poor mass transfer effect, low efficiency, long reaction time, etc., so as to improve production efficiency, speed up reaction rate, The effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
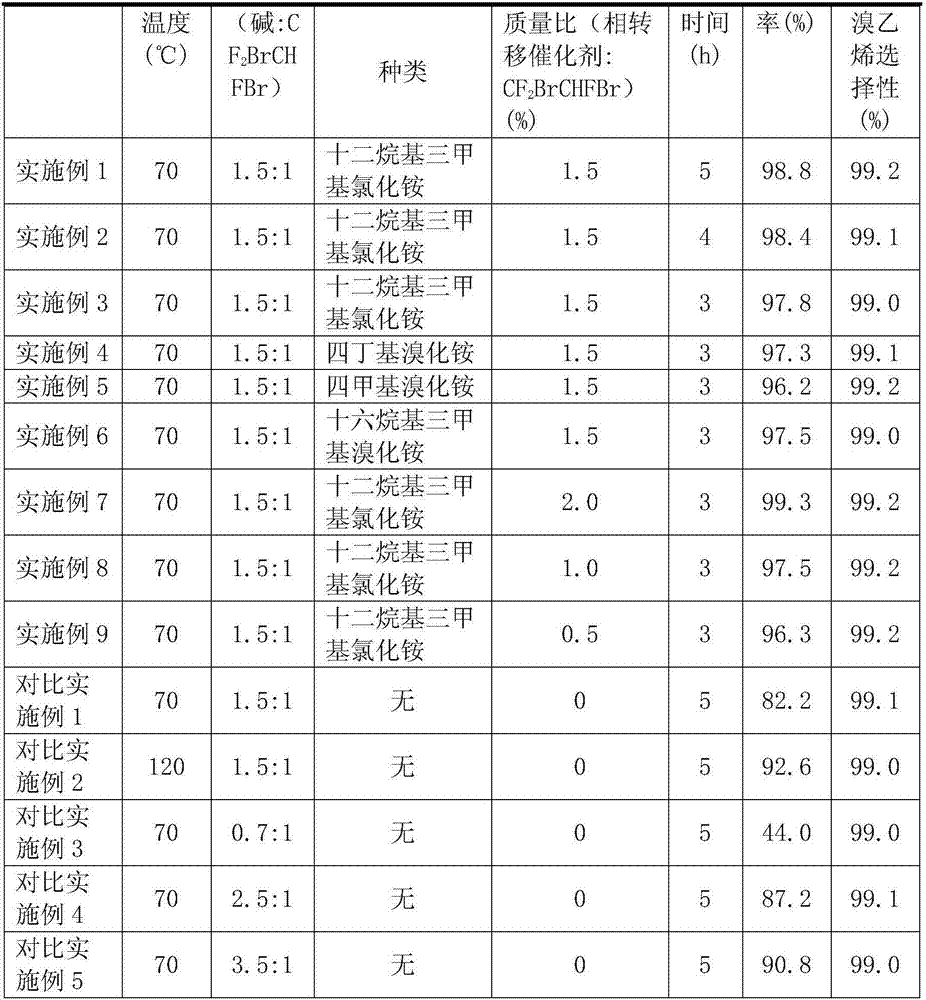
[0033] Put a 250ml three-necked flask in an oil bath, and install 1# cold hydrazine on the three-necked flask for reflux of unreacted raw materials. The collection, the temperature of 2# cold hydrazine is -20 ℃. Add 69g of 30% KOH and 0.9g of dodecyltrimethylammonium chloride into the three-necked flask, and put 60g of raw material 1,2-dibromo-1,1,2-trifluoroethane into the constant pressure funnel (NaOH:CF 2 BrCHFBr=1.5:1 molar ratio). When the temperature of the oil bath was raised to 70°C, the raw material CF was added dropwise to the three-necked flask while stirring. 2 BrCHFBr, continue to keep warm for 5h after the dropwise addition is completed. The reaction results are shown in Table 1.
Embodiment 2
[0035] Put a 250ml three-necked flask in an oil bath, and install 1# cold hydrazine on the three-necked flask for reflux of unreacted raw materials. The collection, the temperature of 2# cold hydrazine is -20 ℃. Add 69g of 30% KOH and 0.9g of dodecyltrimethylammonium chloride into the three-necked flask, and put 60g of raw material 1,2-dibromo-1,1,2-trifluoroethane into the constant pressure funnel (NaOH:CF 2 BrCHFBr=1.5:1 molar ratio). When the temperature of the oil bath was raised to 70°C, the raw material CF was added dropwise to the three-necked flask while stirring. 2 BrCHFBr, continue to keep warm for 4h after the dropwise addition is completed. The reaction results are shown in Table 1.
Embodiment 3
[0037] Put a 250ml three-necked flask in an oil bath, and install 1# cold hydrazine on the three-necked flask for reflux of unreacted raw materials. The collection, the temperature of 2# cold hydrazine is -20 ℃. Add 69g of 30% KOH and 0.9g of dodecyltrimethylammonium chloride into the three-necked flask, and put 60g of raw material 1,2-dibromo-1,1,2-trifluoroethane into the constant pressure funnel (NaOH:CF 2 BrCHFBr=1.5:1 molar ratio). When the temperature of the oil bath was raised to 70°C, the raw material CF was added dropwise to the three-necked flask while stirring. 2 BrCHFBr, continue to keep warm for 3h after the dropwise addition is completed. The reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com