Anti-tumor peptide sourced from hemocyanin of litopenaeus vannamei and application thereof
A technology for tumors and short peptides, which is applied in the field of tumor-suppressing short peptides and their coding genes and applications. It can solve the problems of long peptide chains, difficult chemical synthesis, and difficult purification, and achieve high anti-tumor activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
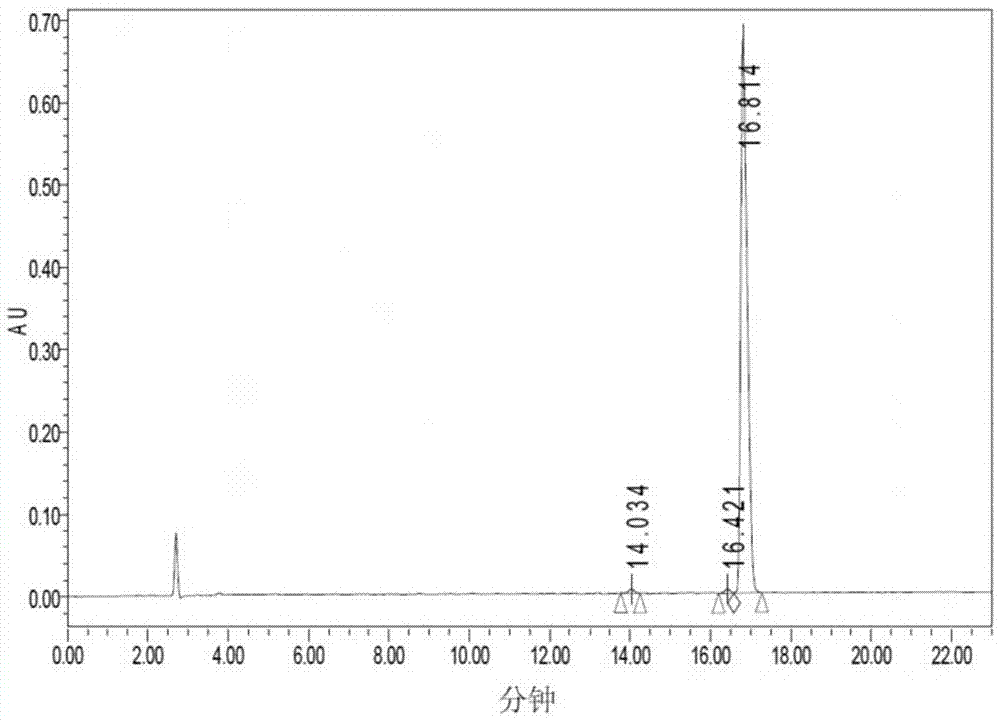
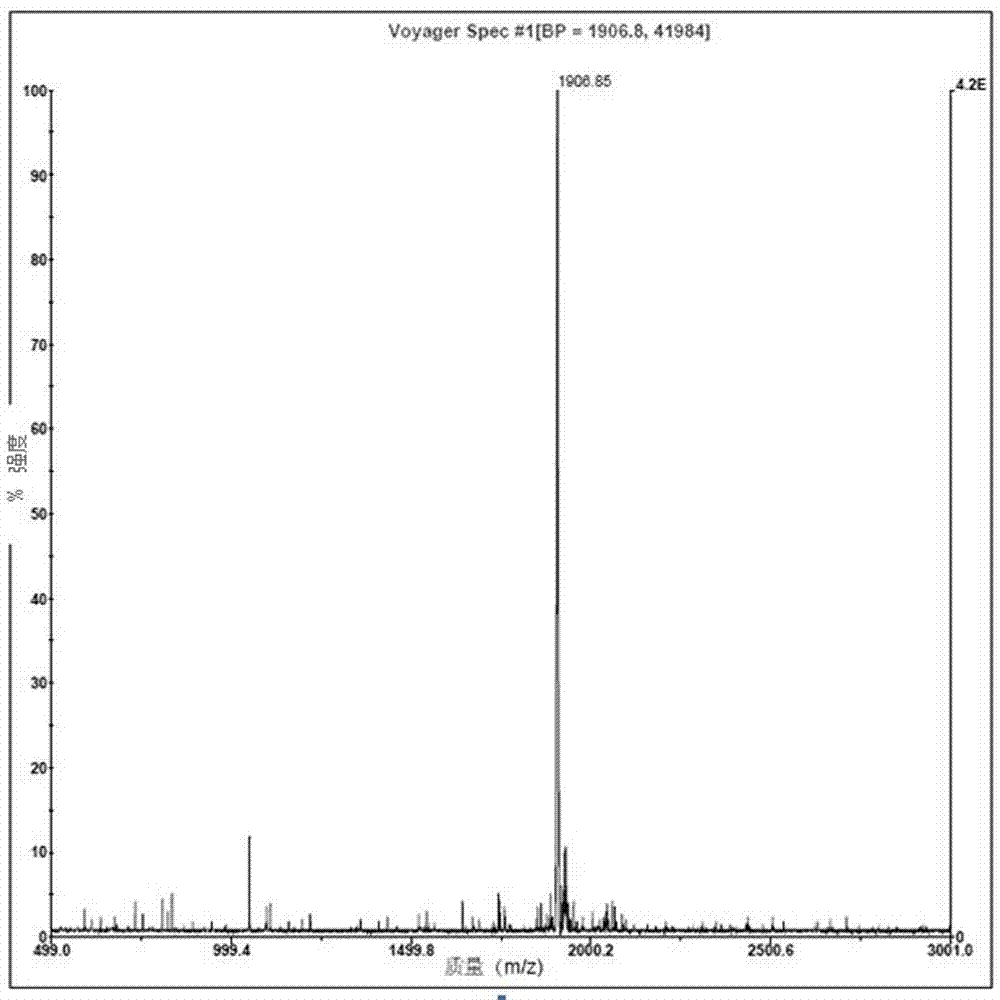
[0026] Example 1: Solid-phase synthesis, cleavage, purification and identification of tumor-suppressing short peptide B1
[0027] The present invention utilizes a polypeptide synthesizer to synthesize the short peptide for inhibiting tumors by a solid-phase synthesis method:
[0028] 1) With DMF as the solvent, the concentration of various α-amino acids protected by Fmoc is 0.25M, the concentration of HBTU solution and HOBt solution is 0.33M, the concentration of piperidine solution is 200ml / L, and the concentration of DIEA solution is 174.2ml / L.
[0029] 2) Weigh 0.05mmol of Fmoc-Ala-Wang resin (functional group content 0.33mmol / g) into a solid-phase reactor, add 8ml of DCM to swell overnight, and remove the solvent under reduced pressure. Add 8ml of piperidine solution with a concentration of 200ml / L, react at room temperature for 5min, and drain; then add 8ml of piperidine solution with a concentration of 200ml / L, react for 20min at room temperature, and drain; wash with 8...
Embodiment 2
[0038] Example 2: Determination of Antitumor Activity of Short Peptide B1 for Inhibiting Tumors
[0039] (1) Use human cervical cancer cells (HeLa) in logarithmic phase of growth, digest and resuspend the cells to obtain a cell suspension, and dilute the cells to 50,000 cells / mL. Add 100 µL to each well of the 96-well plate, put the paved 96-well plate in the incubator, and store at 37°C, 5% CO 2 Incubate overnight (>8 hours) under conditions.
[0040] (2) Use 0.01M pH7.4 PBS to dissolve the short peptide at 1 mg / mL. Dilute the short peptide sample to 50 µg / mL with complete medium. Discard the cell culture medium cultivated overnight, add 100 µL of 50 µg / mL polypeptide to each well, and repeat 3 times in 4 parallels to each well. At the same time, an equal volume of 50 μg / ml 5-FU and 0.01M pH7.4 PBS were used as positive and negative controls, at 5%37 o C conditions were cultured for 18 h.
[0041] (3) Next, discard the culture medium and dry it, add 50 μL of ice-cold 500...
Embodiment 3
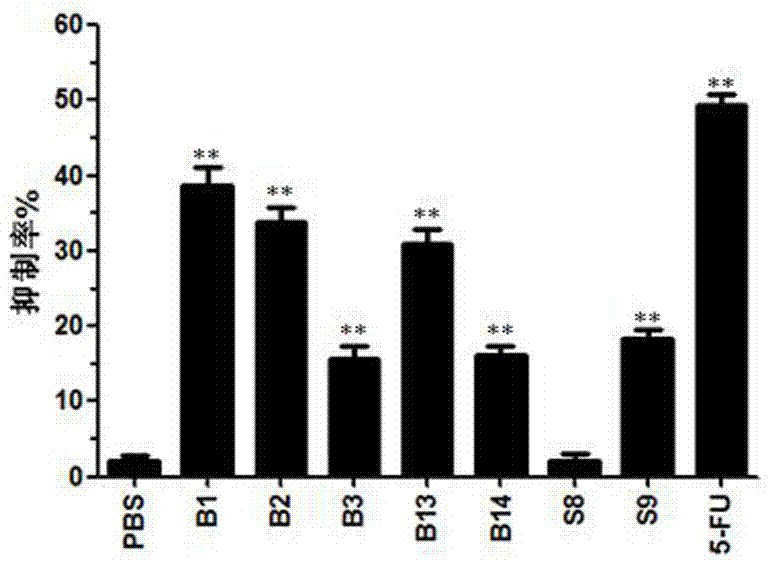
[0046] Example 3: Comparison of the anti-tumor activity of the tumor-suppressing short peptide B1 and other short peptide fragments in hemocyanin
[0047] (1) Synthesize 7 different hemocyanin short peptide fragments and name them respectively (B1, B2, B3, B13, B14, S8, S9,). The short peptide of the present invention is B1.
[0048] (2) Use human cervical cancer cells (HeLa) in the logarithmic phase of growth, digest and resuspend the cells to obtain a cell suspension, and dilute the cells to 50,000 cells / mL. Add 100 µL to each well of the 96-well plate, put the paved 96-well plate in the incubator, and store at 37°C, 5% CO 2 Incubate overnight (>8 hours) under conditions.
[0049] (3) Use 0.01M pH7.4 PBS to dissolve the short peptide at 1mg / mL. Dilute different short peptide samples to 50 µg / mL with complete medium. Discard the cell culture medium cultivated overnight, add 100 µL of 50 µg / mL polypeptide to each well, and repeat 3 times in 4 parallels to each well. At the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com