A process for isobaric ammonia synthesis and co-production of carbon-containing chemicals
An ammonia synthesis and chemical technology, applied in the field of synthetic ammonia, can solve the problems of low concentration of exported ammonia, excess methanol production capacity, low operating rate, etc., and achieve the effects of improving economic benefits, overcoming excess production capacity, and solving low operating rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
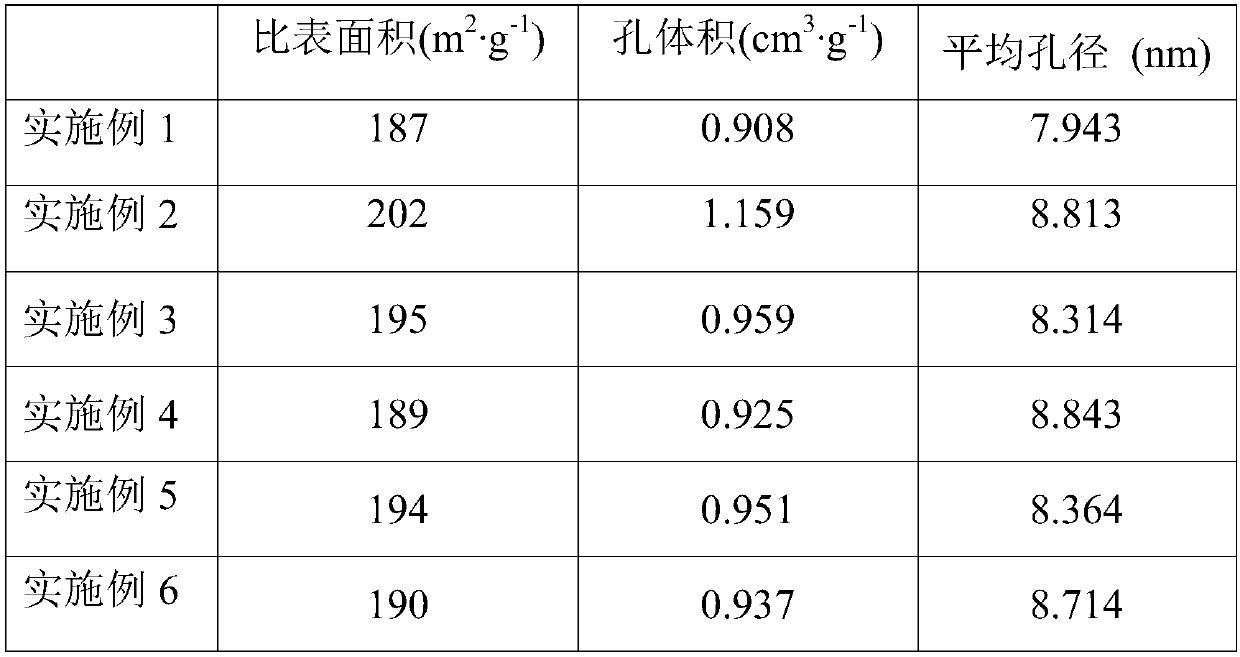
Examples
Embodiment 1
[0051] This embodiment provides a method for preparing a second ruthenium-based catalyst, including the following steps:
[0052] (1) Dissolve 20 g of urea in 100 g of water to prepare a nitrogen-containing solution, and load the nitrogen-containing solution on a magnesium-aluminum hydrotalcite with a magnesium-aluminum molar ratio of 0.6 by an equal volume immersion method, and heat and dry it at 100°C. It is calcined in a nitrogen atmosphere at 190°C for 2 hours to obtain nitrogen-doped magnesium-aluminum hydrotalcite. The doping amount of nitrogen is 5.5% of the mass of the magnesium-aluminum hydrotalcite by mass;
[0053] (2) The nitrogen-doped magnesium-aluminum hydrotalcite was heated at a rate of 15°C / min to 630°C and heat-retained for 1h, and then heated at a rate of 3°C / min to 700°C and heat-retained and baked for 0.75h. It is the catalyst carrier, which contains 30v% spinel phase;
[0054] (3) Heat potassium fluorotantalate and sulfuric acid to above 400°C, and then dilute...
Embodiment 2
[0058] This embodiment provides a method for preparing a second ruthenium-based catalyst, including the following steps:
[0059] (1) Dissolve 15ml of ammonia in 90ml of water to prepare a nitrogen-containing solution, and load the nitrogen-containing solution on a magnesium-aluminum hydrotalcite with a magnesium-aluminum molar ratio of 1.7 by an isometric immersion method, heat and dry it at 80°C, It is calcined in an ammonia atmosphere at 200°C for 0.5h to obtain nitrogen-doped magnesium-aluminum hydrotalcite. The amount of nitrogen doped by mass is 8% of the mass of the magnesium-aluminum hydrotalcite;
[0060] (2) The nitrogen-doped magnesium-aluminum hydrotalcite was heated to 600°C at a rate of 10°C / min and heat-retained and roasted for 3 hours, and then heated to 710°C at a rate of 5°C / min and heat-retained and roasted for 0.5 hours. It is the catalyst carrier. It is determined that the carrier contains 45v% spinel phase, and the pore radius is distributed in the range of 5-...
Embodiment 3
[0065] This embodiment provides a method for preparing a second ruthenium-based catalyst, including the following steps:
[0066] (1) Dissolve 12ml of hydrazine hydrate in 100ml of water to prepare a nitrogen-containing solution, and load the nitrogen-containing solution on a magnesium-aluminum hydrotalcite with a magnesium-aluminum molar ratio of 2.5 by an equal volume immersion method, and heat and dry it at 90°C. Calcined in an ammonia atmosphere at 200°C for 1 hour to obtain nitrogen-doped magnesia-aluminum hydrotalcite. The amount of nitrogen doped by mass is 10% of the mass of the magnesia-aluminum hydrotalcite;
[0067] (2) The nitrogen-doped magnesium-aluminum hydrotalcite was heated at a rate of 5°C / min to 650°C and heat-retained for 2h, and then heated at a rate of 1°C / min to 720°C and heat-retained and baked for 0.5h. It is the catalyst carrier, which contains 37v% spinel phase;
[0068] (3) Heat potassium fluorotantalate and sulfuric acid to above 400°C, and then dilute ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com