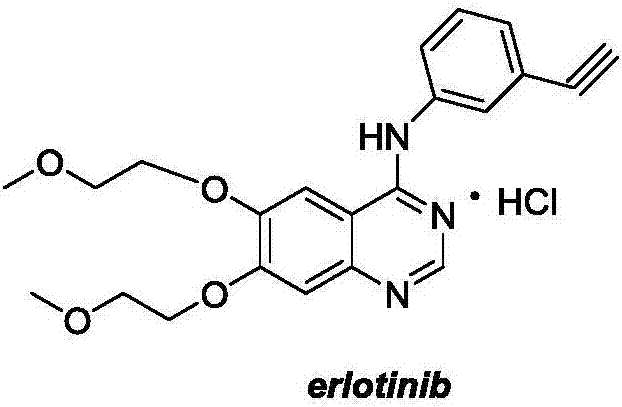
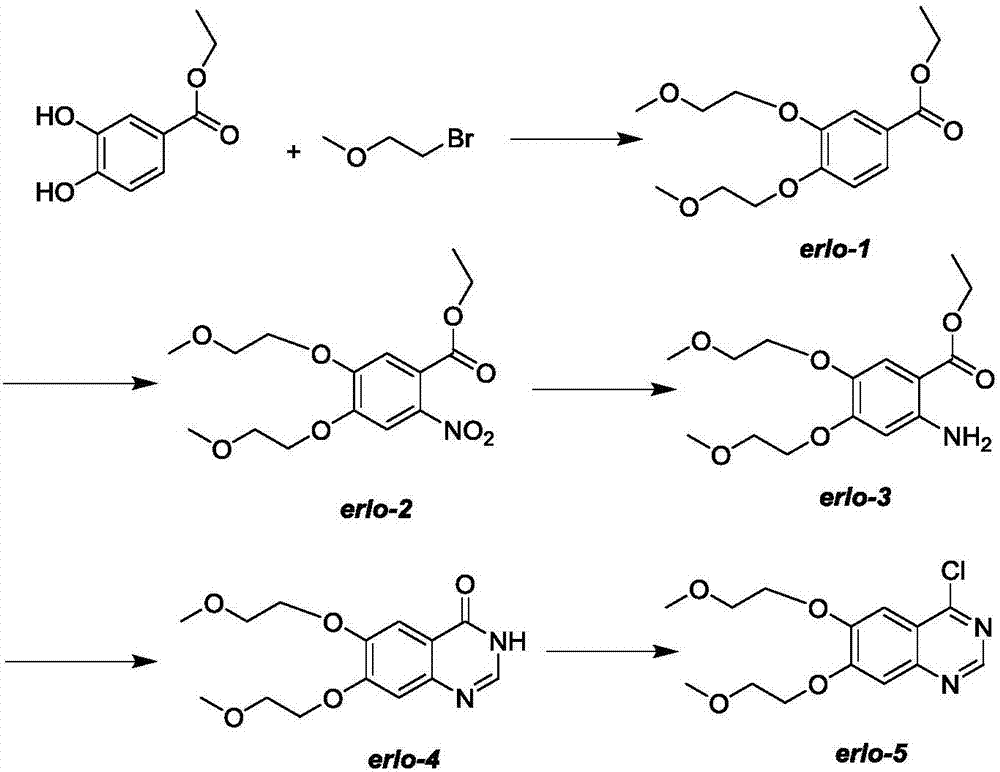
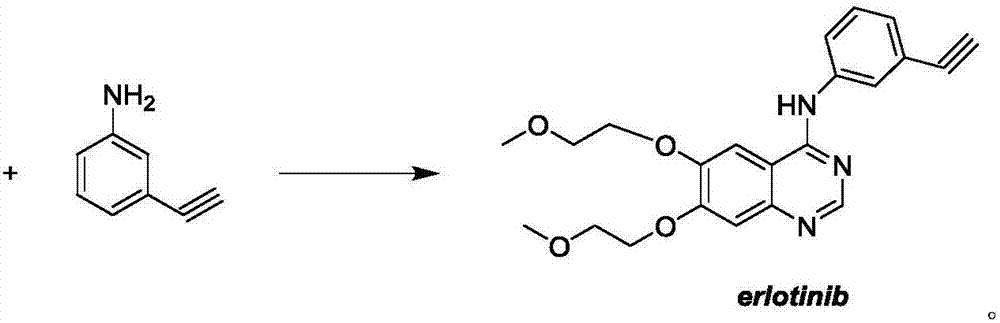
Preparation method of erlotinib intermediate
A technology of intermediates and compounds, which is applied in the field of drug synthesis, can solve the problems of low reaction yield and achieve the effects of high conversion rate, mild reaction conditions and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Weigh compound 1 (285 mg, 1.0 mmol) and dissolve it in DMF (10 mL), add paraformaldehyde (300 mg, 10 mmol) and a catalytic amount of boron trifluoride diethyl ether at room temperature, heat to 50 ° C, stir the reaction, and detect the reaction by TLC The raw material (compound 1) completely disappeared (about 5h of reaction), stop heating and cool down to room temperature, pour the reaction solution into ice water (about 100mL), stir for 5-10min, filter cake with suction, add 20mL of acetonitrile to the filter cake, heat for 50 ℃ until the solid was completely dissolved, and naturally cooled to room temperature to crystallize, suction filtered, the filter cake was washed twice with acetonitrile, and dried in vacuo to obtain 292 mg of a white solid (the yield was about 98.9%), which was compound 2 (ESI-MS (m / z ): 296.11[M+H] + , 318.09[M+Na] + , 1 H NMR (400MHz, CDCl 3 ):δ8.23(s,1H,H-2),7.50(s,1H,H-5),7.18(s,1H,H-8),4.28-4.20(m,8H,CH 2 ×4),3.43(s,3H,CH ...
Embodiment 2
[0036] Weigh compound 1 (285 mg, 1.0 mmol) and dissolve it in THF (10 mL), add paraformaldehyde (600 mg, 20 mmol) and a catalytic amount of boron trifluoride diethyl ether at room temperature, heat to 60 ° C, stir the reaction, and detect the reaction by TLC The raw material (compound 1) completely disappeared (about 3h of reaction), stop heating and cool down to room temperature, pour the reaction solution into ice water (about 100mL), stir for 5-10min, filter cake with suction, add 20mL of acetonitrile to the filter cake, heat for 50 ℃ until the solid was completely dissolved, and naturally cooled to room temperature to crystallize, suction filtered, the filter cake was washed twice with acetonitrile, and dried in vacuo to obtain 293 mg of a white solid (the yield was about 99.2%), which was compound 2 (structure confirmation data and Example 1 consistent).
Embodiment 3
[0038] Weigh compound 1 (285mg, 1.0mmol) and dissolve it in DMF (10mL), add paraformaldehyde (300mg, 10mmol) at room temperature, heat to 50°C, stir and react (about 5h), TLC detection shows that the compound in the reaction solution 1 is still the main point, that is, it almost reacts without adding boron trifluoride ether.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com