A genetic engineering strain used for producing phenazine-1-carboxamide, a preparing method thereof and uses of the strain
A technology of genetically engineered strains and formamide, which is applied in the field of genetic engineering, can solve the problems of complex metabolic regulation network and difficulty in further improving the yield of secondary metabolites, etc., and achieves the effects of good stability, promoting industrial and agricultural applications, and increasing yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
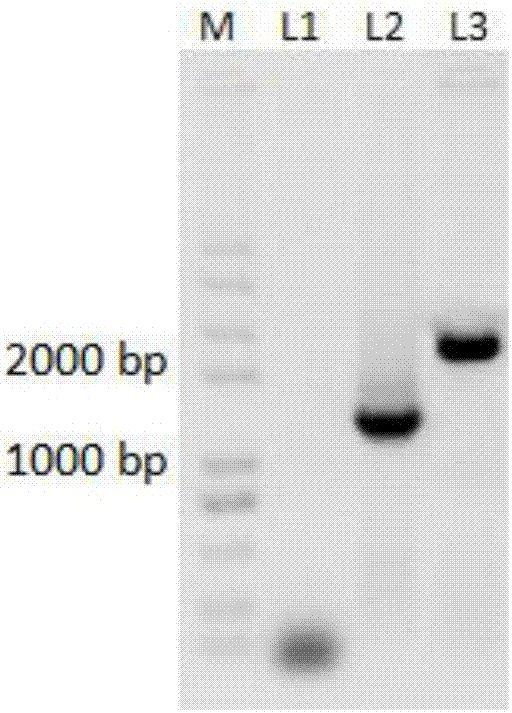
[0038] This embodiment relates to a method of using the HT66 genome as a template to prepare the HT66ΔpykA genetically engineered strain by the insertion mutagenesis method, including the following steps:
[0039] 1. Primer Design
[0040] Using the pykA gene sequence as a reference, primers were designed and synthesized using primer 5. The primer sequences are as follows, and the underlines indicate the cleavage sites of restriction endonucleases Hind III and Sac I.
[0041] pykA1:CC AAGCTTATGTCCGTCCGTCGTACCAA (SEQ ID NO. 3)
[0042] pykA2:AC GAGCTC TCAGACCATTGGGTCGCCA (SEQ ID NO. 4)
[0043] Using the plasmid pBbB5k as a template, primers for the Kan resistance gene (Kan) were designed and synthesized. The sequences of the primers are as follows, and the underline indicates the cleavage site of the restriction endonuclease Xho I.
[0044] pBb-Kan-F:CC CTCGAG GGAATTGCCAGCTGGGGCGC SEQ ID NO.5
[0045] pBb-Kan-R:CC CTCGAG TCAGAAGAACTCGTCAAGAAG SEQ ID NO. 6
[0046] ...
Embodiment 2
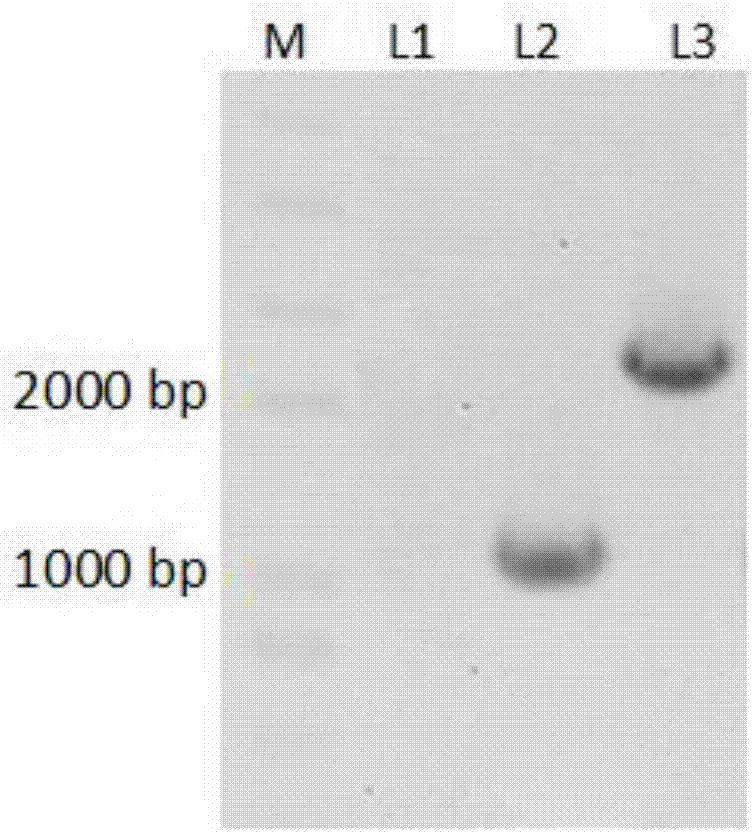
[0053] This embodiment relates to the preparation of a high-yield PCN genetically engineered strain. The starting strain P3 is a high-yield PCN strain obtained by multiple mutations of Pseudomonas chloropinus HT66. Specifically include the following steps:
[0054] 1. Primer design:
[0055] Using the upstream and downstream sequences of the pykA gene as a reference, the knockout primers were designed and synthesized using primer 5. The primer sequences are as follows (the underline indicates the XbaI and HindIII restriction sites):
[0056] pykAF1:CCGGGGATCC TCTAGA AAGATCGTTACAACGCGGTCG (SEQ ID NO. 7)
[0057] pykAR1:TGGTACGACGGACGGACATG (SEQ ID NO. 8)
[0058] pykAF2:TGTCCGTCCGTCGTACCAACCCAATGGTCTGAGCCACC (SEQ ID NO. 9)
[0059] pykAR2:GGCCAGTGCC AAGCTT CGAGTTCGGTTCCAGCCTG (SEQ ID NO. 10)
[0060] 2. Recombinant plasmid construction:
[0061] Using the P3 genome as a template, the upstream and downstream homology arms of pykA were amplified by PCR using pykAF1, pykA...
Embodiment 3
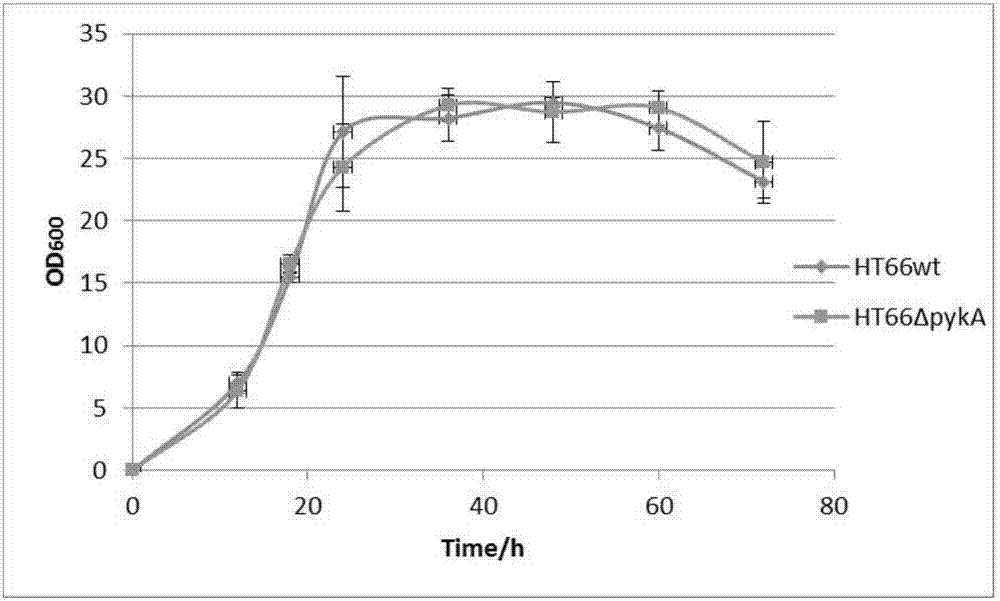
[0066] This embodiment relates to a method for synthesizing PCN using the genetically engineered bacterial strain prepared in Example 1, comprising the following steps:
[0067] Bacterial strains HT66ΔpykA and HT66wt (wild strain HT66 as a control) were respectively activated twice on KB solid plates, then cultured in 5 mL KB liquid medium, cultured to logarithmic phase, and transferred to 60 mL KB culture medium at a ratio of 2%. The basic Erlenmeyer flask was used for fermentation. The fermentation conditions were 28° C., 180 rpm. Samples were taken regularly, and the PCN yield of the strain was determined by HPLC.
[0068] HPLC detection conditions:
[0069] The mobile phase is acetonitrile-25mM ammonium acetate, the chromatographic column is WondaSilC18-WRreversephasecolumn (5μm; 4.6×250mm, Shimadzu, Japan), the detection wavelength is 254nm, the detection conditions: 0-2min, 8% acetonitrile-92% 25mM ammonium acetate, 2- In 20 minutes, the concentration of acetonitrile ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com