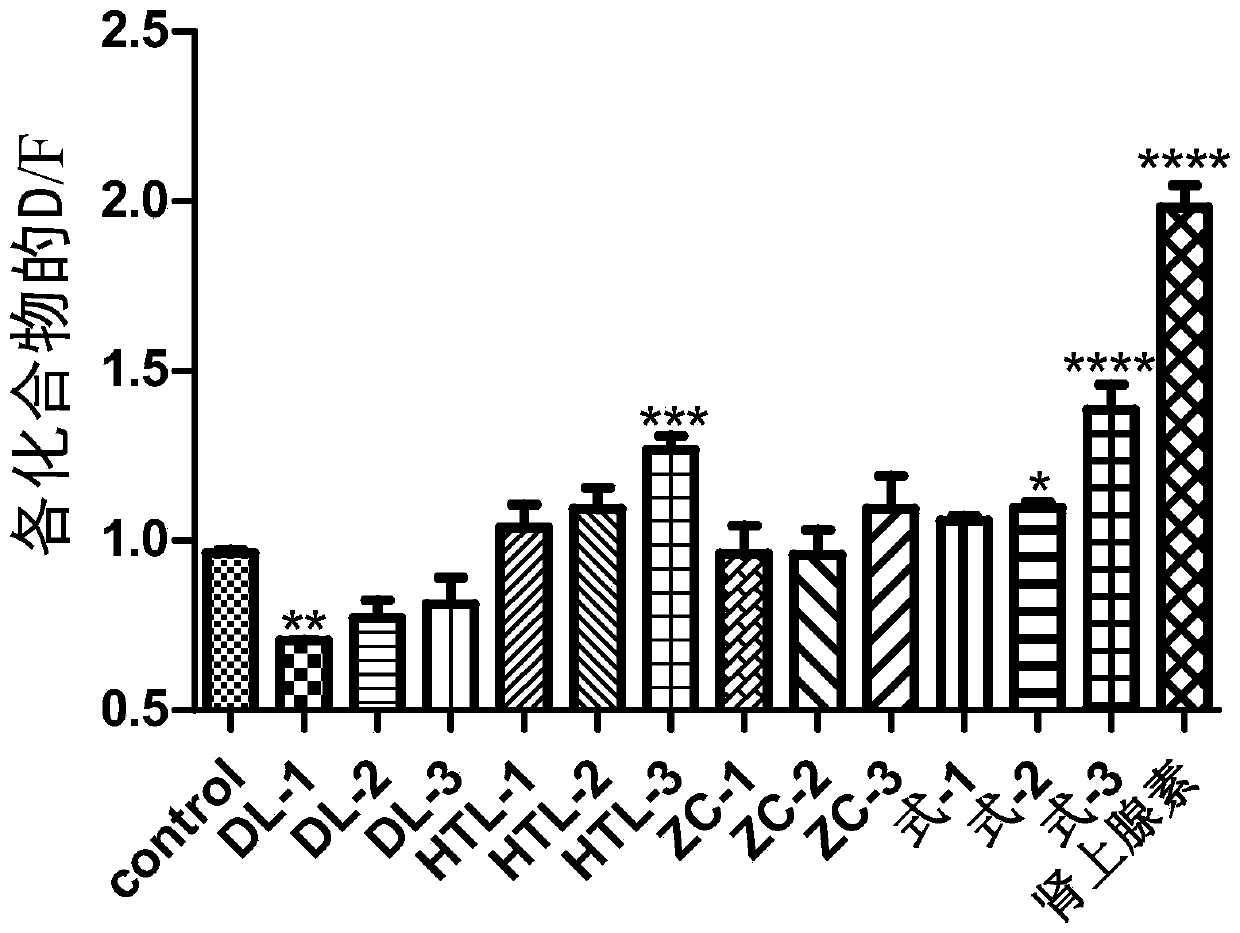
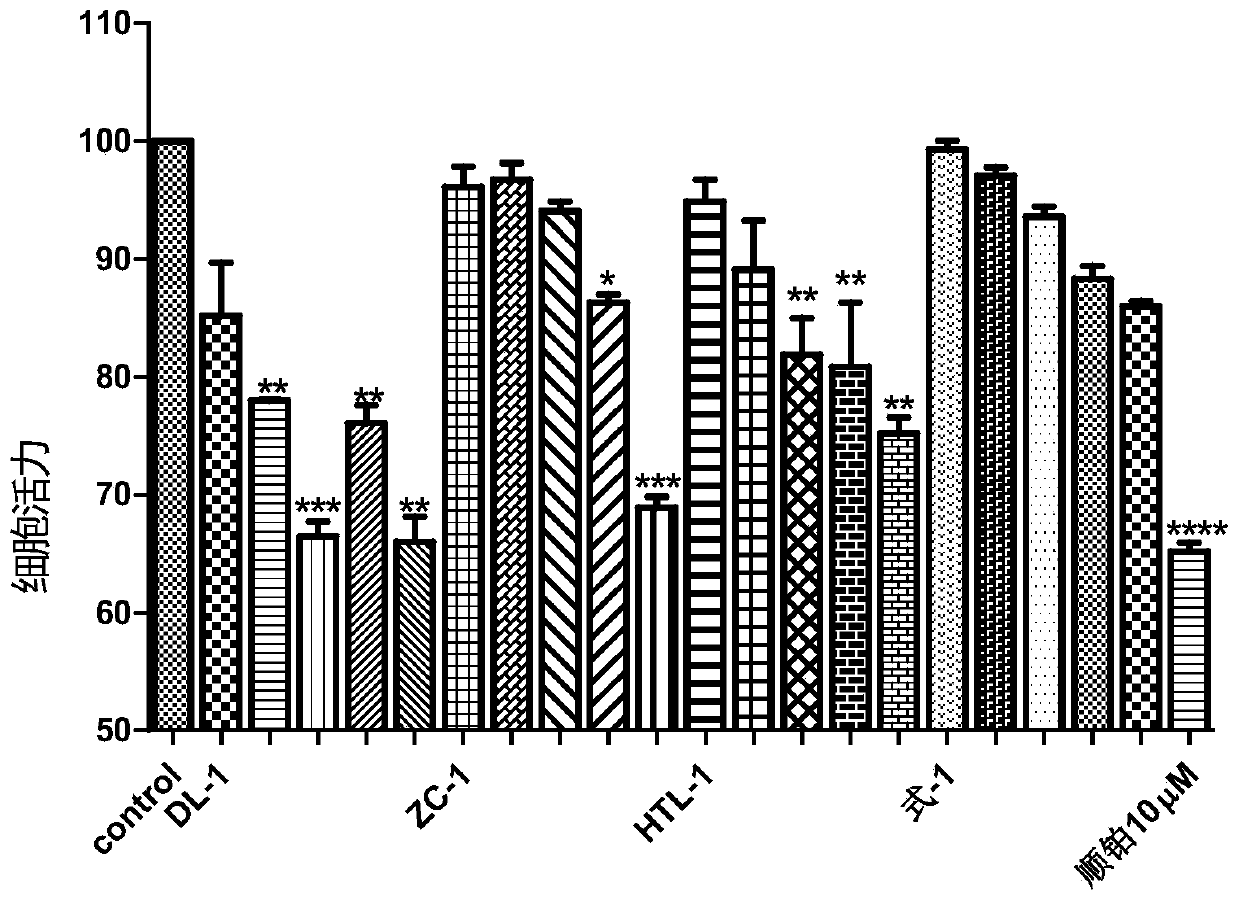
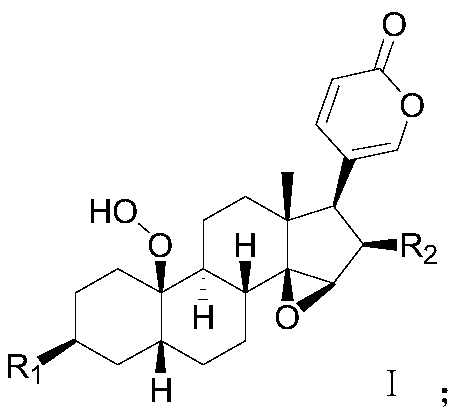
A kind of steroid compound and its use
A technology of compounds and steroids, applied in the field of steroids, can solve the problems of strong cardiotoxicity and cytotoxicity, affecting clinical application, etc., and achieve the effect of cardiotonic effect, significant effect, and weak cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the preparation of formula II compound
[0043] a) Using 2 kg of dried toad venom pulp as raw material, add 8 times the amount of 95wt% methanol aqueous solution, and carry out reflux extraction for 3 times, each time for 2 hours; combine the filtrates, concentrate under reduced pressure until there is no alcohol smell, and obtain a liquid extract; The paste was suspended in water, and extracted with dichloromethane until the extract was colorless; the extracts were combined, concentrated under reduced pressure to extract, and a crude extract was obtained;
[0044] b) The crude extract is separated by silica gel column chromatography, and the volume ratio is 10:10:1, 8:8:1, 6:6:1, 4:4:1, 2:2:1, 1: A 1:1 mixed solvent of petroleum ether-dichloromethane-methanol was used for gradient elution, each eluent was evaporated under reduced pressure and then analyzed by TLC to obtain eight components according to the order of elution, denoted as Fr.E1~ E8;
[0045...
Embodiment 2
[0052] Embodiment 2: the preparation of formula III compound
[0053] a) Using 2 kg of dried toad venom pulp as raw material, add 8 times the amount of 60wt% methanol aqueous solution, and carry out reflux extraction for 3 times, each time for 2 hours; combine the filtrates, concentrate under reduced pressure until there is no alcohol smell, and obtain a liquid extract; The paste was suspended in water, and extracted with dichloromethane until the extract was colorless; the extracts were combined, concentrated under reduced pressure to extract, and a crude extract was obtained;
[0054] b) The crude extract is separated by silica gel column chromatography, and the volume ratio is 10:10:1, 8:8:1, 6:6:1, 4:4:1, 2:2:1, 1: A 1:1 mixed solvent of petroleum ether-dichloromethane-methanol was used for gradient elution, each eluent was evaporated under reduced pressure and then analyzed by TLC to obtain eight components according to the order of elution, denoted as Fr.E1~ E8;
[005...
Embodiment 3
[0062] Embodiment 3: the preparation of formula IV compound
[0063] a) Using 2 kg of dried toad venom pulp as raw material, add 8 times the amount of 60wt% methanol aqueous solution, and carry out reflux extraction for 3 times, each time for 2 hours; combine the filtrates, concentrate under reduced pressure until there is no alcohol smell, and obtain a liquid extract; The paste was suspended in water, and extracted with dichloromethane until the extract was colorless; the extracts were combined, concentrated under reduced pressure to extract, and a crude extract was obtained;
[0064] b) The crude extract is separated by silica gel column chromatography, and the volume ratio is 10:10:1, 8:8:1, 6:6:1, 4:4:1, 2:2:1, 1: A 1:1 mixed solvent of petroleum ether-dichloromethane-methanol was used for gradient elution, each eluent was evaporated under reduced pressure and then analyzed by TLC to obtain eight components according to the order of elution, denoted as Fr.E1~ E8;
[0065...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com