Reptilase and its production process and application
A technology of hemocoagulase and snake venom, which is applied in the field of snake venom hemocoagulase and its production method and application, to achieve the effects of safe clinical use, shortening coagulation time, and shortening bleeding time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, the production of hemocoagulase and hemocoagulase pharmaceutical preparation of white-browed snake venom of the present invention
[0037] Take 20 g of white-browed snake venom crystals and dissolve them in 1000 ml of 0.9% NaCl injection, centrifuge at 3000 rpm for 10 minutes, and discard the precipitate. Adjust the pH of the supernatant to 6.5, and then dilute to 1000 ml with 0.9% NaCl injection. To this solution, 670 ml of saturated ammonium sulfate solution was added, and the mixture was left at room temperature for 1 hour, then centrifuged at 3000 rpm for 10 minutes, and the precipitate was discarded. Add 552 ml of saturated ammonium sulfate solution to the supernatant, let it stand at room temperature for 1 hour, centrifuge the mixture at 3000 rpm for 10 minutes, discard the supernatant precipitate and dissolve it in 1000 ml of distilled water, and adjust the pH to 5.0. 7.7 ml of 90% phenol was added to this solution, and the mixture was left at room...
Embodiment 2
[0043] Embodiment 2, Determination of hemagglutinase-like activity of the white-browed snake venom of the present invention
[0044] The hemagglutinase of the white-browed snake venom of the present invention has a hemagglutinase-like effect and a thromboplastin-like effect, and the hemagglutinase-like effect of the white-browed snake venom on human fibrinogen is observed by adding EDTA to inhibit its thromboplastin-like effect effect.
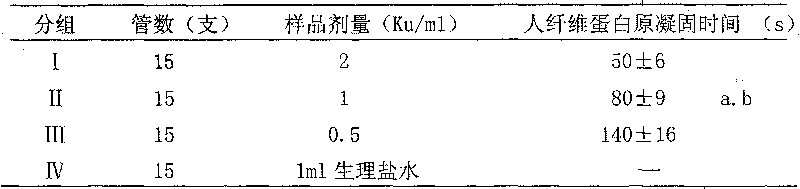
[0045] Add Hemocoagulase sample 1ml (every sample is dissolved with 1ml 5 × 10-3mol / L EDTA) of the present invention in test tube, be divided into 4 groups, I, II, III are respectively Hemocoagulase high, middle , low-dose group, the doses are 2Ku / ml, 1Ku / ml, 0.5Ku / ml respectively, IV group is the blank group (add physiological saline 1ml), each tube acts on 37 ℃ water bath for 5 minutes after 5 hours, and then adds to each tube 0.1% human fibrinogen 1ml, observe and record the coagulation time of human fibrinogen. The results are shown in T...
Embodiment 3
[0050] Example 3, Determination of Thromboplastin-Like Activity of Hemocoagulase from Snake Venom of the Present Invention
[0051] The hemagglutinase-like effect of the white-browed snake venom of the present invention has a hemagglutinase-like effect and a thromboplastin-like effect, and the hemagglutase-like effect of the white-browed snake venom is observed by adding DFP (diisopropylfluorophosphoric acid) to inhibit the hemagglutase-like effect of the white-browed snake venom. Thromboplastin-like effect.
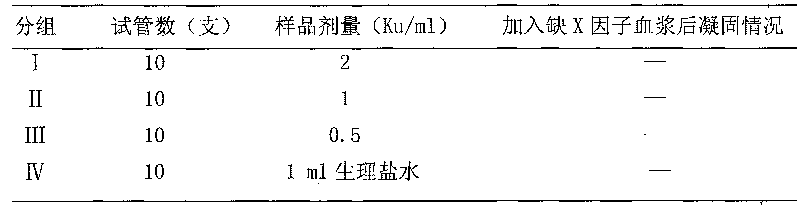
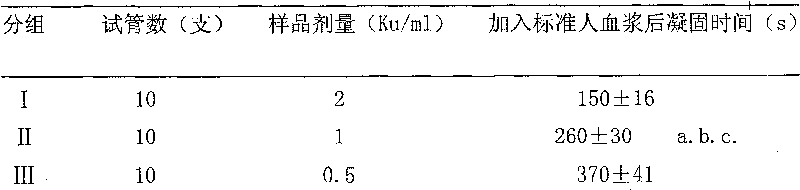
[0052] Add Hemocoagulase sample 1ml (every sample is dissolved with 2 * 10-3mol / L DFP solution) of the present invention in test tube, be divided into 4 groups, I, II, III group are respectively Hemocoagulase high, Hemocoagulase of Hemodon venom of the present invention, In the middle and low dose groups, the doses were 2Ku / ml, 1Ku / ml, and 0.5Ku / ml respectively. The IV group was the blank control group (with normal saline). Add 1ml of factor X-deficient standard plasma ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com