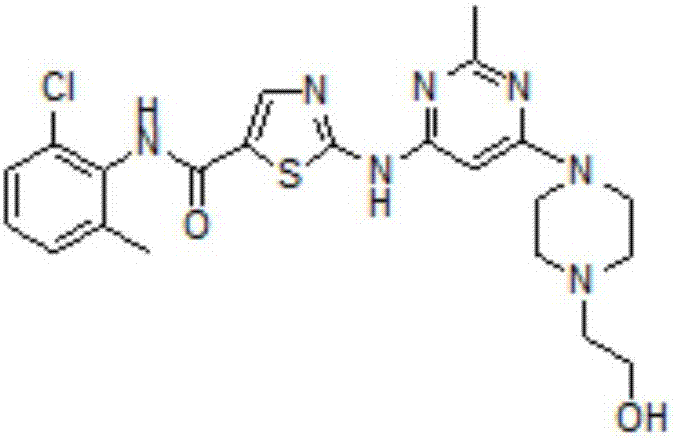
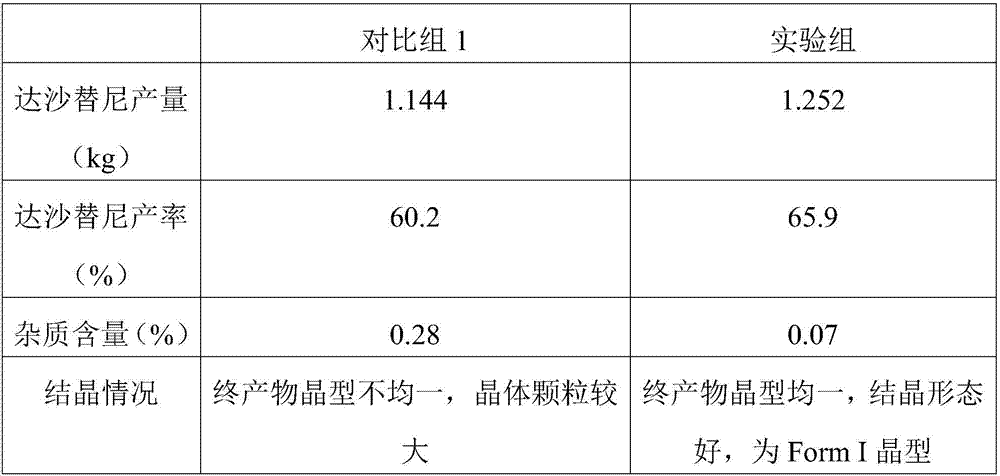
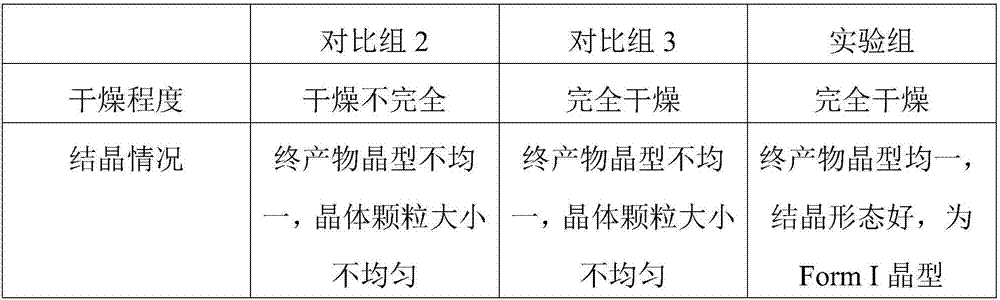
Preparation method of dasatinib
A technology of dasatinib and methyl phenyl, applied in the field of preparation of dasatinib, can solve the problems of high raw material cost, low product yield and high production cost, and achieve the effects of high yield and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] A preparation method of dasatinib, comprising the following steps:
[0057] (1) Preparation of N-(2-chloro-6-methylphenyl)-2-[(6-chloro-2-methyl-4-pyrimidinyl)amino]-5-thiazole carboxamide: at room temperature, the 1.50kg 2-amino-N-(2-chloro-6-methylphenyl)-5-thiazole amide, 1.11kg 4,6-dichloro-2-methylpyrimidine were added to the 50L reaction kettle, and 13.35kg Tetrahydrofuran, nitrogen protection, stirring to cool down to -5 ° C ~ 10 ° C, the temperature of the reaction solution is kept at -5 ° C ~ 10 ° C, adding sodium tert-butoxide solids to the reaction solution in batches, the total amount of sodium tert-butoxide solids is 2.13 kg, the addition method is to add once every 20-30min, each time no more than 10% of the total amount and no more than 200g at most. After the solution, the temperature was raised to 10°C to 30°C, and the reaction liquid was stirred until the HPLC detection of 2-amino-N-(2-chloro-6-methylphenyl)-5-thiazole amide / N-(2-chloro-6- The amount...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com