Method for predicting residue of ciprofloxacin in pig body by physiologically based pharmacokinetic model
A technology of physiological pharmacokinetics and ciprofloxacin, applied in the field of food safety risk assessment, can solve the problems of false positives, high cost and low cost, and achieve the effect of reducing economic loss and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for predicting ciprofloxacin residues in pigs by a physiological pharmacokinetic model, comprising the following steps:
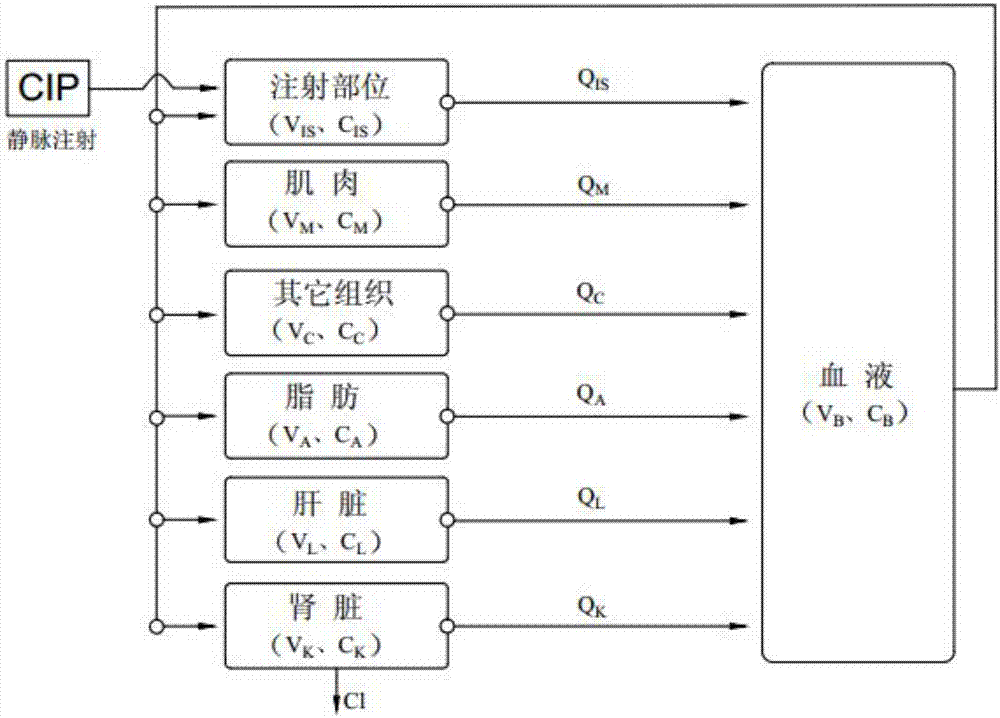
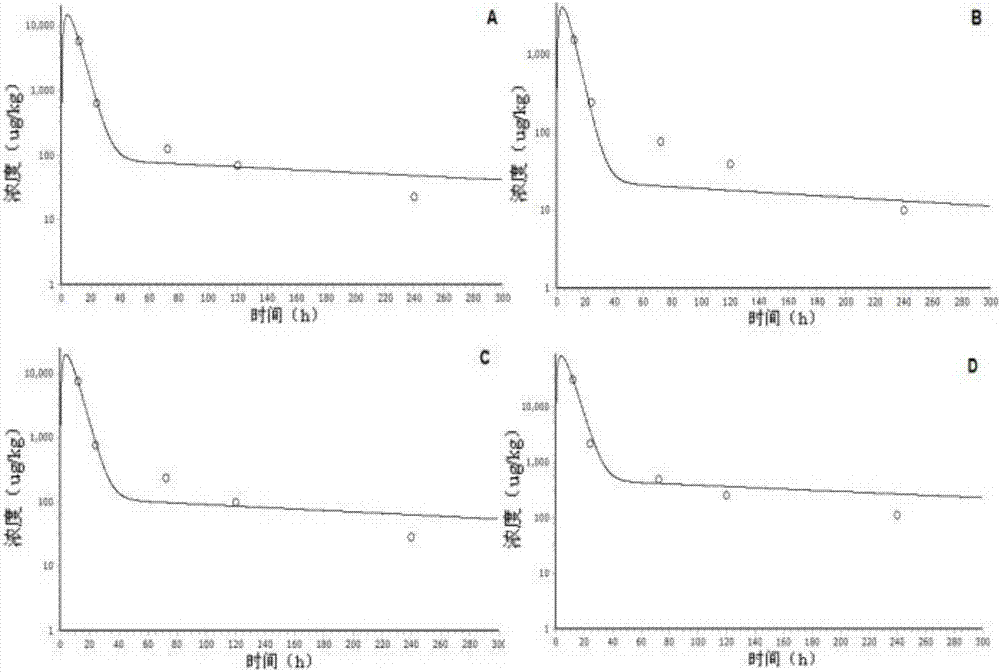
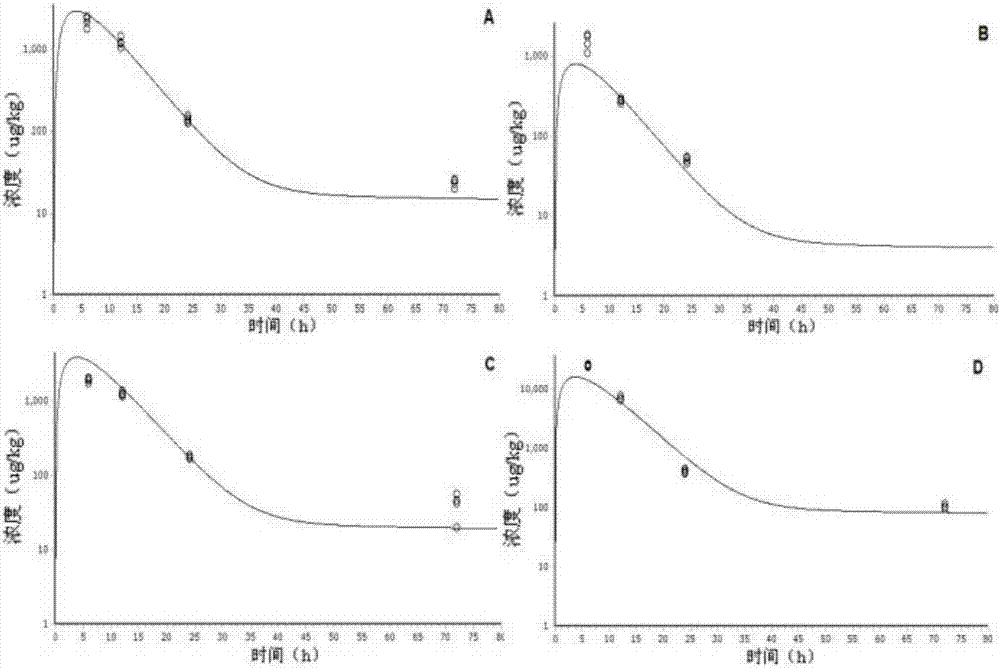
[0035] Ciprofloxacin is a small molecule substance. For drug residues, we focus on the drug concentration in edible tissues and vital organs, so the model established does not include organs with special physiological barriers. Ciprofloxacin has high fat solubility and strong permeability, and its distribution and transport in the body mainly depends on the blood perfusion rate. Therefore, it can be assumed that the absorption of ciprofloxacin obeys the first-order rate after intramuscular injection, and the distribution in various tissues and organs The speed and degree mainly depend on the blood flow flowing through the tissues and organs, which obeys the speed-limiting distribution of blood flow, is excreted by the kidneys, and obeys the first-order rate. The chamber of the physiological pharmacokinetic model described in the present inve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com