Olaparib dihydrate and preparation method thereof
A technology of dihydrate and purified water, which is applied in the field of medicine, can solve problems such as inability to break through, and achieve the effects of improved solubility, simple and easy-to-operate preparation method, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1, the preparation of olaparib dihydrate
[0057] 1) Dissolve 100 g of the crude product of olaparib in (1500 ml) in a mixed solvent of dimethyl sulfoxide and water (dimethyl sulfoxide: water=2:1), stir and heat at a speed of 220 rpm to make the crude product completely Dissolve until the solution is clear and filter;
[0058] 2) Slowly lower the temperature of the solution obtained above at a rate of 3°C every 10 minutes. When it drops to 0°C, add 6000ml of pre-cooled purified water to the solution at a flow rate of 2.0mL / min until the crystals appear, and continue to cool down to -20 Crystallize at ℃, heat and stir until the crystallization is complete, and grow the crystal for 2 hours;
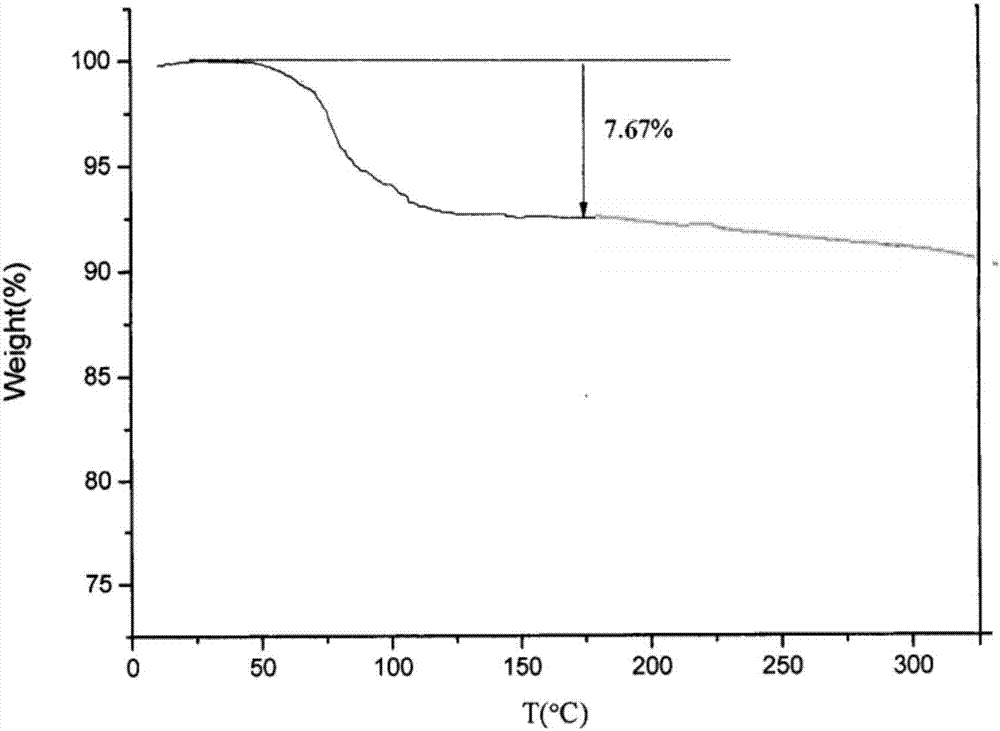
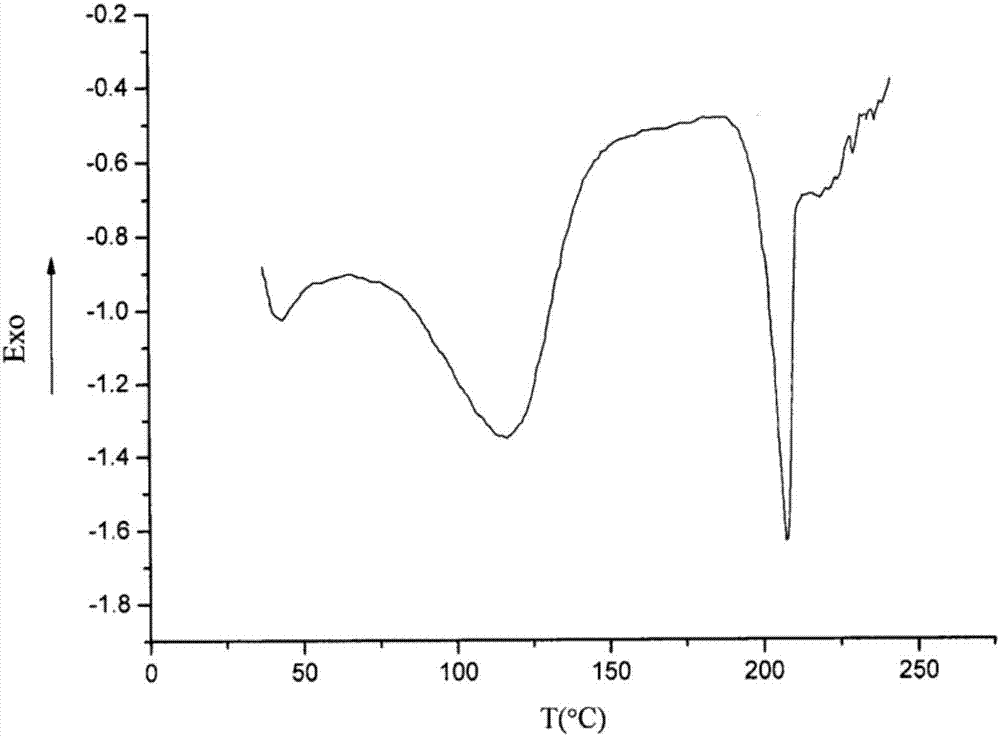
[0059] 3) The crystals were collected by suction filtration, washed with a small amount of purified water, and dried in vacuum at 50° C. for 4 hours to obtain 99.90 g of white crystalline powder with a yield of 99.90% and a purity of 99.98%.
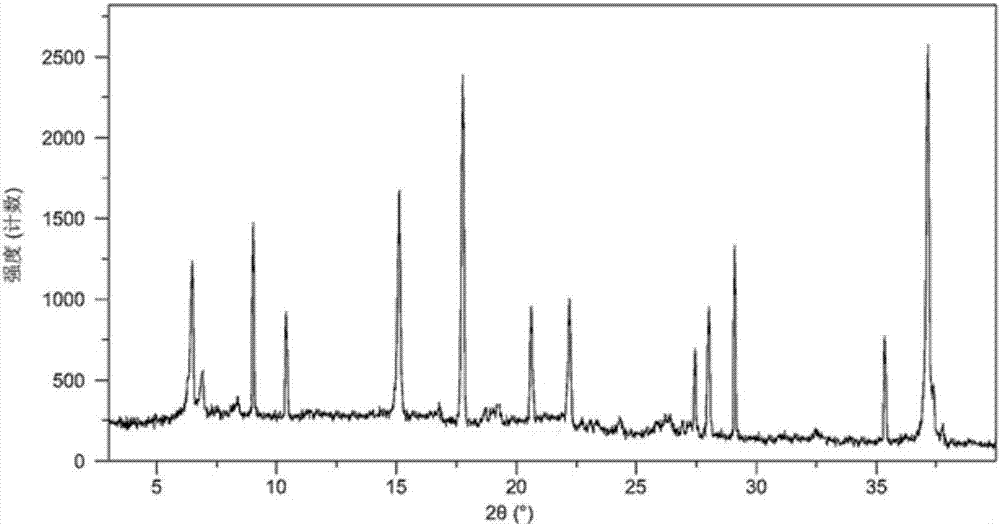
[0060] The X-ray powder dif...
Embodiment 2
[0061] Embodiment 2, the preparation of olaparib dihydrate
[0062] 1) Dissolve 100 g of the crude product of olaparib in a mixed solvent of 2000 ml of dimethyl sulfoxide and water (dimethyl sulfoxide: water=3:1), stir and heat at a speed of 250 rpm to completely dissolve the crude product, To the solution clarification, filter;
[0063] 2) Slowly lower the temperature of the solution obtained above at a rate of 1°C every 10 minutes. When it drops to -5°C, add 6000ml of pre-cooled purified water to the solution at a flow rate of 2.2mL / min until the crystals appear, and continue to cool down to - Crystallize at 15°C, heat and stir until the crystallization is complete, and grow the crystal for 4 hours;
[0064] 3) The crystals were collected by suction filtration, washed with a small amount of purified water, and dried in vacuum at 45° C. for 5 hours to obtain 99.91 g of white crystalline powder with a yield of 99.91% and a purity of 99.99%.
[0065] The X-ray powder diffract...
Embodiment 3
[0066] Embodiment 3, the preparation of olaparib dihydrate
[0067] 1) Dissolve 100 g of the crude product of olaparib in a mixed solvent of 1800 ml of dimethyl sulfoxide and water (dimethyl sulfoxide: water=3:1), stir and heat at a speed of 240 rpm to completely dissolve the crude product, To the solution clarification, filter;
[0068] 2) Slowly lower the temperature of the solution obtained above at a rate of 2°C every 10 minutes. When it drops to -3°C, add 6300ml of pre-cooled purified water to the solution at a flow rate of 2.5mL / min until the crystals appear, and continue to cool down to - Crystallize at 15°C, heat and stir until the crystallization is complete, and grow the crystal for 3 hours;
[0069] 3) The crystals were collected by suction filtration, washed with a small amount of purified water, and dried in vacuum at 45° C. for 5 hours to obtain 99.94 g of white crystalline powder with a yield of 99.94% and a purity of 99.99%.
[0070] The X-ray powder diffract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com