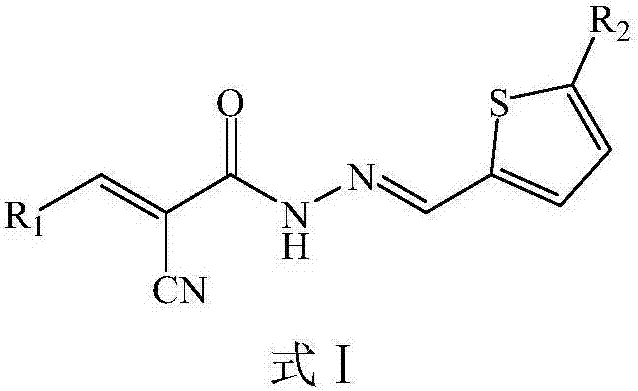
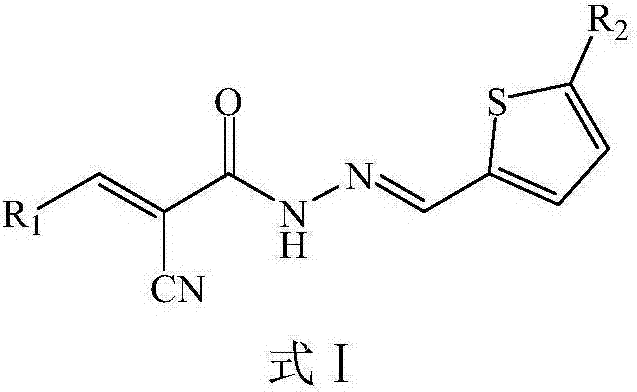
N-(2-Thienyl)methylene-2-cyano-3-heterocyclic acrylhydrazine derivative and application thereof
A technology of alkyl and compound, which is applied in the field of heterocyclic Schiff base compounds, can solve the problems of the use of heterocyclic Schiff base compounds as antioxidants and agricultural pesticides, and achieve excellent antioxidant performance and novel structure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
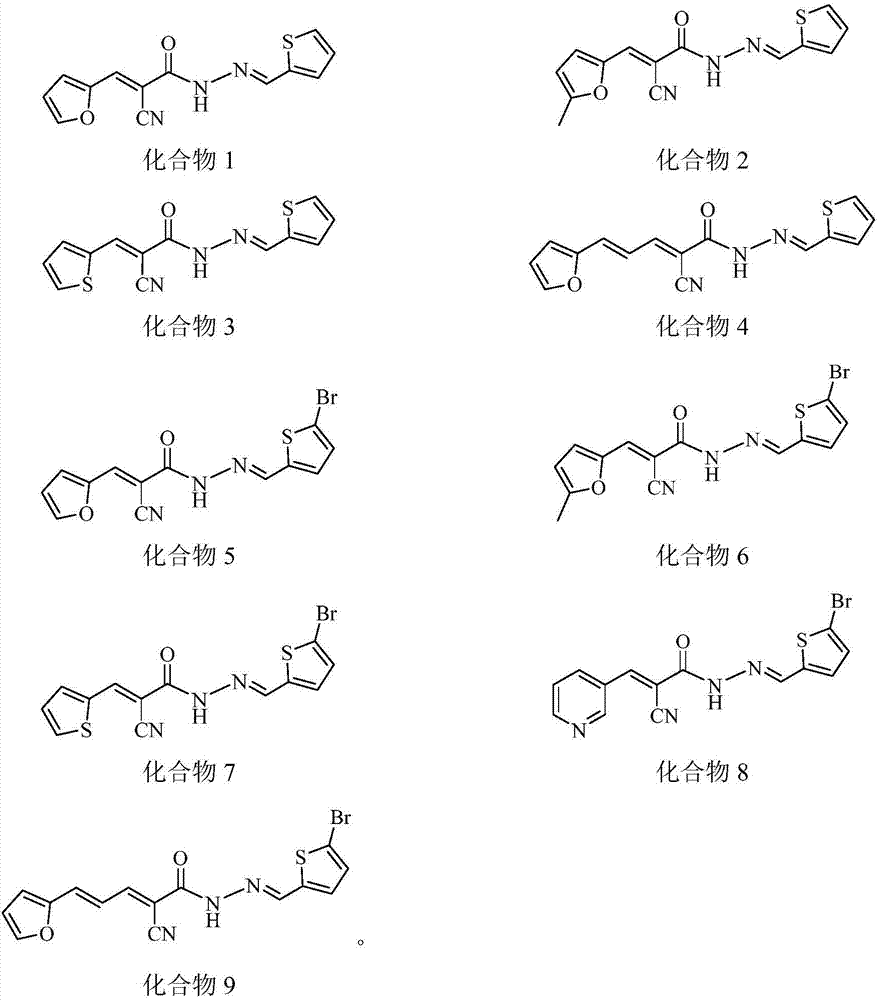
[0036] The synthesis of embodiment 1 compound 1~9
[0037] Synthesis principle:
[0038]
[0039] 1. Synthesis of compound 1
[0040] (1) Synthesis of intermediates
[0041] Put 0.01mol of cyanoacetylhydrazide in a three-necked flask, add 15mL of dichloromethane as a solvent, 3mL of acetic acid as a catalyst, and stir at room temperature. Take 0.01mol of thiophene-2-carboxaldehyde and add it to 5mL of dichloromethane. After fully dissolving, add it dropwise into a three-necked flask with a constant pressure dropping funnel, react for 5 hours, and detect the end point with a thin-layer silica gel plate (TLC). The reaction solution was placed in a beaker and washed repeatedly with distilled water until neutral. Liquid separation and vacuum distillation, suction filtration and drying to obtain the intermediate.
[0042] (2) Synthesis of the target compound
[0043] Take 0.01 mol of the intermediate and place it in a three-neck flask, add 15 mL of absolute ethanol as a sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com