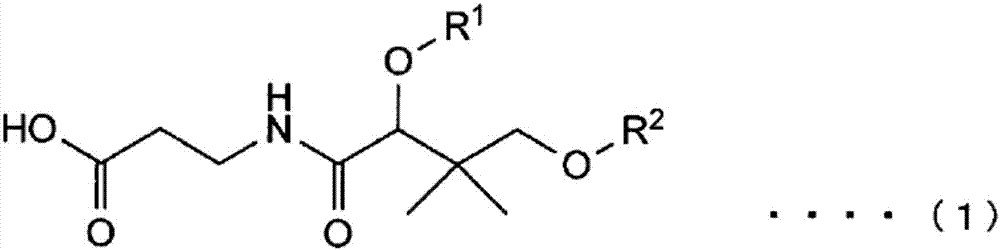
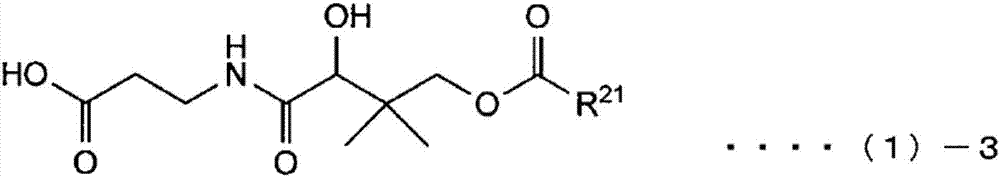
Compound, salt of compound, external agent for skin, cosmetic, and food additive
一种化合物、通式的技术,应用在化妆品、化妆品配制品、护理皮肤的制剂等方向,能够解决效果不充分等问题,达到吸收性高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
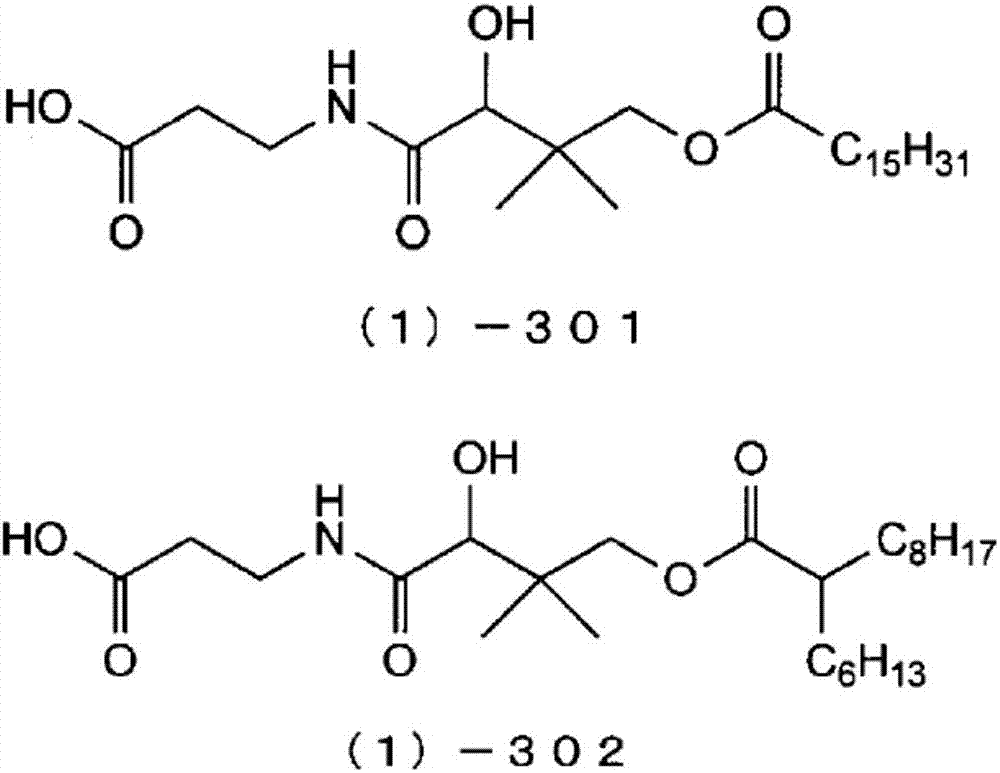
[0206] According to the following steps, the compound shown in the following formula (1)-301 (in the general formula (1), R 1 is a hydrogen atom, R 2 The compound which is a linear hexadecanoyl group is hereinafter abbreviated as "compound (1)-301").
[0207]
[0208] Calcium pantothenate (manufactured by Tokyo Chemical Industry Co., Ltd., 19.1 g, 40.1 mmol), pyridine (manufactured by Wako Pure Chemical Industries, Ltd., 19.0 g, 240 mmol) and 4-dimethylaminopyridine (DMAP) (manufactured by Wako Pure Chemical Industries, Ltd., 0.49 g, 4.0 mmol) was added to tetrahydrofuran (THF) (manufactured by Wako Pure Chemical Industries, Ltd., 560 mL) and stirred, and palmitoyl chloride (manufactured by Tokyo Chemical Industry Co., Ltd., 22.6 g, 82.2 mmol) was added dropwise at room temperature (25° C.) over 3 hours, and further stirred at room temperature for 3.5 hours after the dropwise addition was completed. At this time, a part of the reaction liquid was sampled, analyzed by HPLC...
Embodiment 2
[0216] According to the following steps, the compound shown in the following formula (1)-302 (in the general formula (1), R 1 is a hydrogen atom, R 2 A branched 2-hexyldecanoyl compound is hereinafter abbreviated as "compound (1)-302").
[0217]
[0218] As an acylating agent, it reacted similarly to Example 1 except having used 2-hexyldecanoyl chloride (made by Nippon Seika Co., Ltd.) instead of palmitoyl chloride, and having made reaction temperature 50 degreeC instead of room temperature. A part of the reaction solution was sampled, analyzed by HPLC, and quantified. As a result, the yield of the target compound (1)-302 was 13.4%. Thereafter, post-treatment of the reaction solution, extraction of the crude product, and purification by column chromatography were carried out in the same manner as in Example 1, whereby the target compound (1)-302 was obtained as a pale yellow oil. (IUPAC name: 3-[N-(4-(2-hexyldecanoyl)oxy-3,3-dimethyl-2-hydroxybutyryl)amino]propanoic acid)...
reference example 1
[0224] According to the following procedure, a compound represented by the following formula (9)-101 (hereinafter, abbreviated as "compound (9)-101") was produced.
[0225]
[0226] As the acylating agent, instead of palmitoyl chloride, decanoyl chloride (manufactured by Tokyo Chemical Industry Co., Ltd.) was used, and the reaction was carried out in the same manner as in Example 1. A part of the reaction solution was sampled, analyzed by HPLC, and quantified. As a result, the yield of (9)-101, which is the target substance, was 24.4%. Hereinafter, post-treatment of the reaction solution, extraction of the crude product, and purification by column chromatography were carried out in the same manner as in Example 1 to obtain the target compound (9)-101 (IUPAC Name: 3-[N-(4-decanoyloxy-3,3-dimethyl-2-hydroxybutyryl)amino]propanoic acid) (yield 3.41g (9.14mmol), isolated yield 11.4%) .
[0227] The obtained product is compound (9)-101 through 1 H-NMR and 13 It was confirmed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com