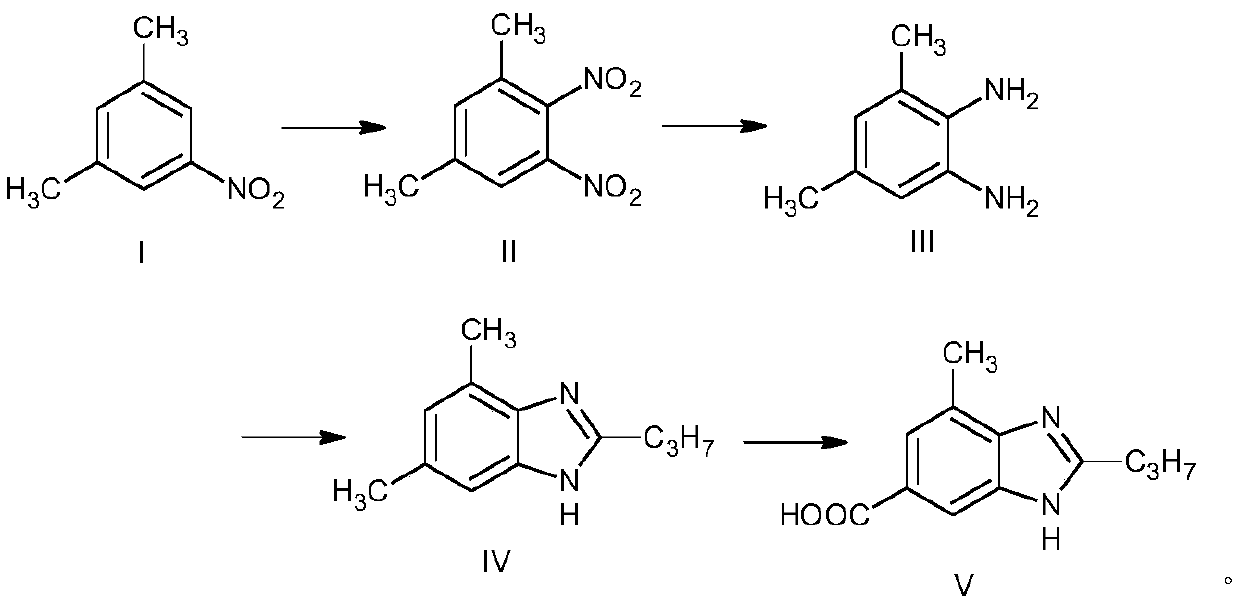
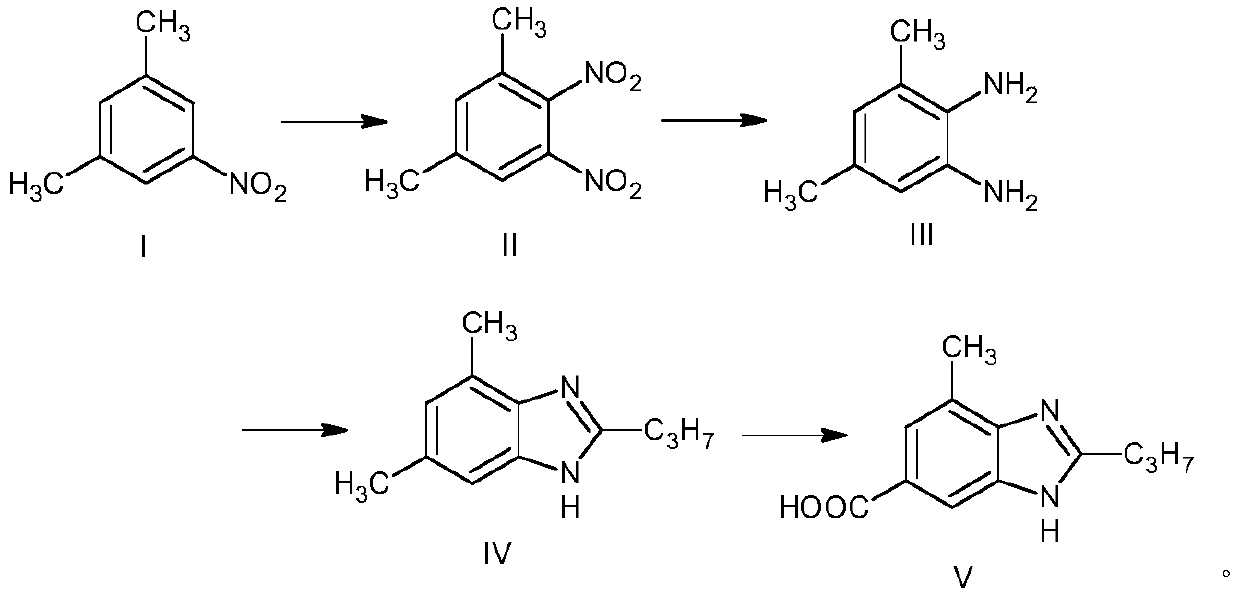
The synthetic method of 2-n-propyl-4-methylbenzimidazole-6-carboxylic acid
A technology of methylbenzimidazole and dimethylbenzimidazole, applied in the field of synthesis of telmisartan intermediate 2-n-propyl-4-methylbenzimidazole-6-carboxylic acid, achieving less pollution , easy to obtain raw materials, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The synthetic method of the present invention is carried out in four steps:
[0020] In the first step, 3,5-dimethylnitrobenzene is used as raw material, nitrated by nitric acid / concentrated sulfuric acid, hydrolyzed, and filtered to generate intermediate product II 3,5-dimethyl-1,2-dinitrobenzene.
[0021] Specifically: prepare a mixed acid with 95% fuming nitric acid and 98% concentrated sulfuric acid, and store it at 0°C; add 98% concentrated sulfuric acid and 3,5-dimethylnitrobenzene into the reaction vessel, and cool it under stirring To -10°C, add dropwise the mixed acid prepared by 95% fuming nitric acid and 98% concentrated sulfuric acid, and control the feeding rate so that the reaction temperature is not higher than -5°C. After the dropwise addition, continue to stir in the ice bath for 3 hours, slowly add the reaction mixture to crushed ice for hydrolysis, keep the temperature not exceeding 5°C, and stir vigorously until a large amount of light yellow solid p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com