Synthetic method for 2-chloro-4-amino-6,7-dimethoxy quinazoline
A technology of dimethoxyquinazoline and synthesis method, which is applied in the synthesis field of 2-chloro-4-amino-6,7-dimethoxyquinazoline, can solve the problem of insufficient reaction of reactants and target product Instability, complex synthesis method and other problems, to achieve the effect of complete reaction, stable yield and simplified reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
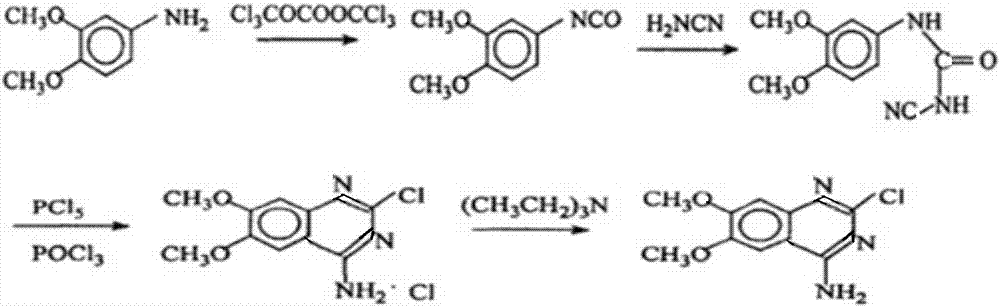
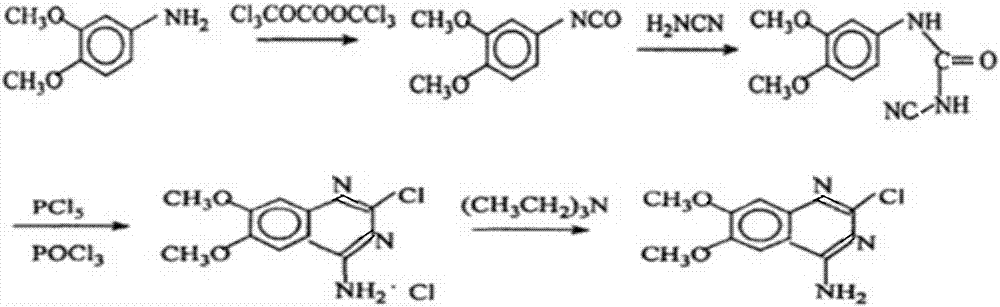
[0037] The synthetic method of 2-chloro-4-amino-6,7-dimethoxyquinazoline of the present invention comprises the steps:
[0038] 1) Preparation of 3,4-dimethoxyphenyl isocyanate
[0039] (1) First dissolve 3,4-dimethoxyaniline and dichloroethane and then heat to generate 3,4-dimethoxyaniline solution, the quality of 3,4-dimethoxyaniline and dichloroethane The ratio is 3:10, mixing in this ratio can improve the reaction efficiency of 3,4-dimethoxyaniline and dichloroethane, make the reaction of reactants more thorough, and reduce the waste of raw materials;
[0040] (2) Then add triphosgene and dichloroethane into the first reaction flask, stir and dissolve, lower the temperature to within 10°C, add 3,4-dimethoxyaniline solution dropwise into the first reaction flask, ~ 2 hours to finish dripping;
[0041] (3) heating and refluxing for 3h, reclaiming ethylene dichloride to obtain 3,4-dimethoxyphenyl isocyanate;
[0042] 2) Preparation of 3,4-dimethoxyphenylcyanuria
[0043] ...
Embodiment 1
[0060] 1) Preparation of 3,4-dimethoxyphenyl isocyanate
[0061] (1) At first 124g of 3,4-dimethoxyaniline and 400ml of ethylene dichloride were dissolved and then heated to generate 3,4-dimethoxyaniline solution;
[0062] (2) Then add triphosgene and 400ml of dichloroethane into the first reaction flask, stir and dissolve, lower the temperature to within 10°C, add the 3,4-dimethoxyaniline solution dropwise into the first reaction flask, Finish dripping within 1 to 2 hours;
[0063] (3) warming up and refluxing for 3h, reclaiming ethylene dichloride to obtain 3,4-dimethoxyphenyl isocyanate with a yield of 0.94;
[0064] 2) Preparation of 3,4-dimethoxyphenylcyanuria
[0065] (1) at first concentration is that 50% cyanamide 150g and concentration are 50% lye 200g to add in the second reaction bottle, reduce temperature to within 3 ℃;
[0066] (2) Add 3,4-dimethoxyphenyl isocyanate dropwise to the second reaction flask, and raise the temperature to 30° C. after the dropwise ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com