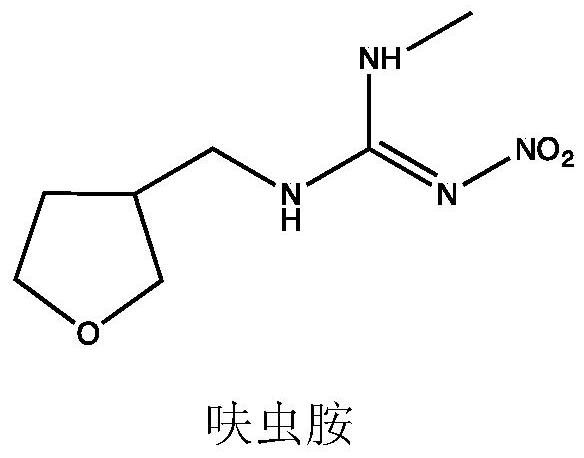
A kind of synthetic method of tetrahydro-3-furan methanol
A technology of furanmethanol and tetrahydrofuran, which is applied in the synthesis field of tetrahydro-3-furanmethanol, can solve the problems of cumbersome synthesis route, many "three wastes", difficult operation, etc., and achieves simple post-processing, less three wastes" and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
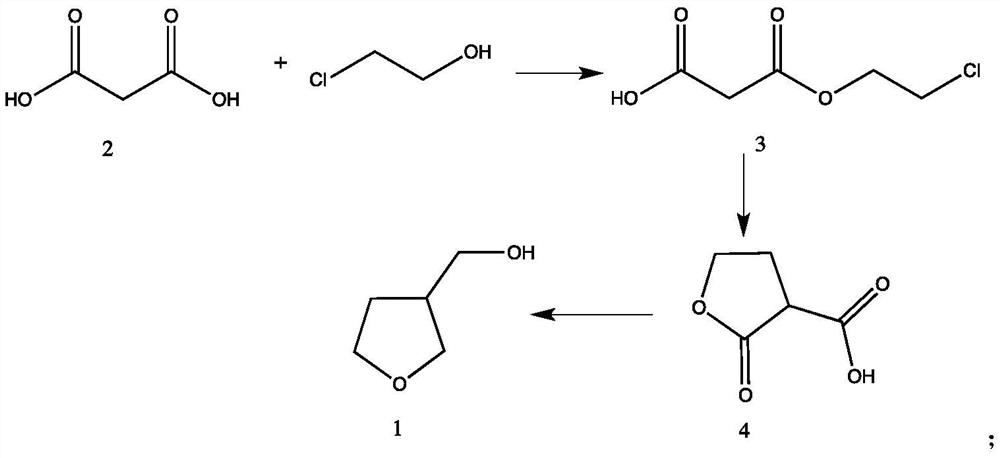
[0027] A synthetic method of tetrahydro-3-furanmethanol, the specific preparation method is as follows:
[0028] At 0°C, slowly add chloroethanol (80.5g, 1mol) dropwise into a solution of malonic acid (104g, 1mol) and 300g of tetrahydrofuran for 2 hours, then add 1g of concentrated sulfuric acid, and return to room temperature at 25°C for reaction After 16 hours, tetrahydrofuran was recovered by distillation to obtain 160 g of monochloroethyl malonate with a yield of 96.6% and a purity of 91% by GC analysis.
[0029] Disperse NaH (40g, 1mol) in 200g of toluene, stir at 0°C for 1h, then dissolve monochloroethyl malonate (150g, 0.9mol) in 200g of toluene, and slowly add it dropwise to In the reaction bottle, return to room temperature (25°C) and react for 12 hours, cool down to 0°C, adjust the pH to 3-4 with concentrated hydrochloric acid, filter to remove inorganic salts, and distill the mother liquor to recover toluene to obtain 3-formic acid-γ-butyrolactone , 105.5g, yield 9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com