A crystal form and b crystal form of triptolide, and preparation method and use thereof
A technology of tripteryglide and its crystal form, which is applied in the field of medicinal chemistry, can solve the problems of low bioavailability, poor water solubility of tripteryne, hindering the development of finished medicine, etc., and achieve simple preparation methods, high solubility, and improved physical and chemical properties. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Take 20mg of tripterine raw material, add 1mL of acetonitrile solvent, suspend at room temperature for 2 days, centrifuge and take powdery solid, after vacuum drying, put it in an oven at 150°C and heat to obtain the A crystal form of tripterine. For the red powder.
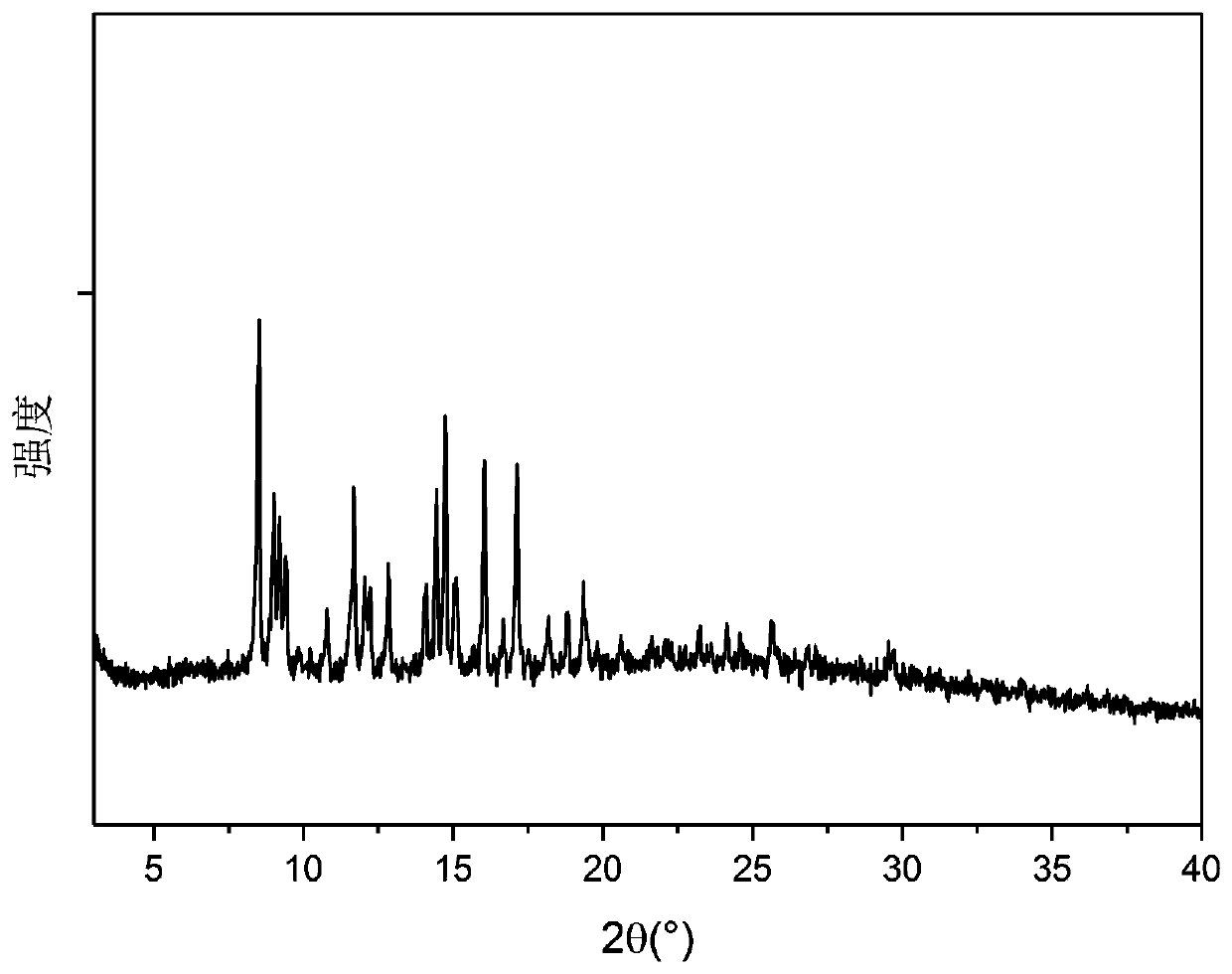
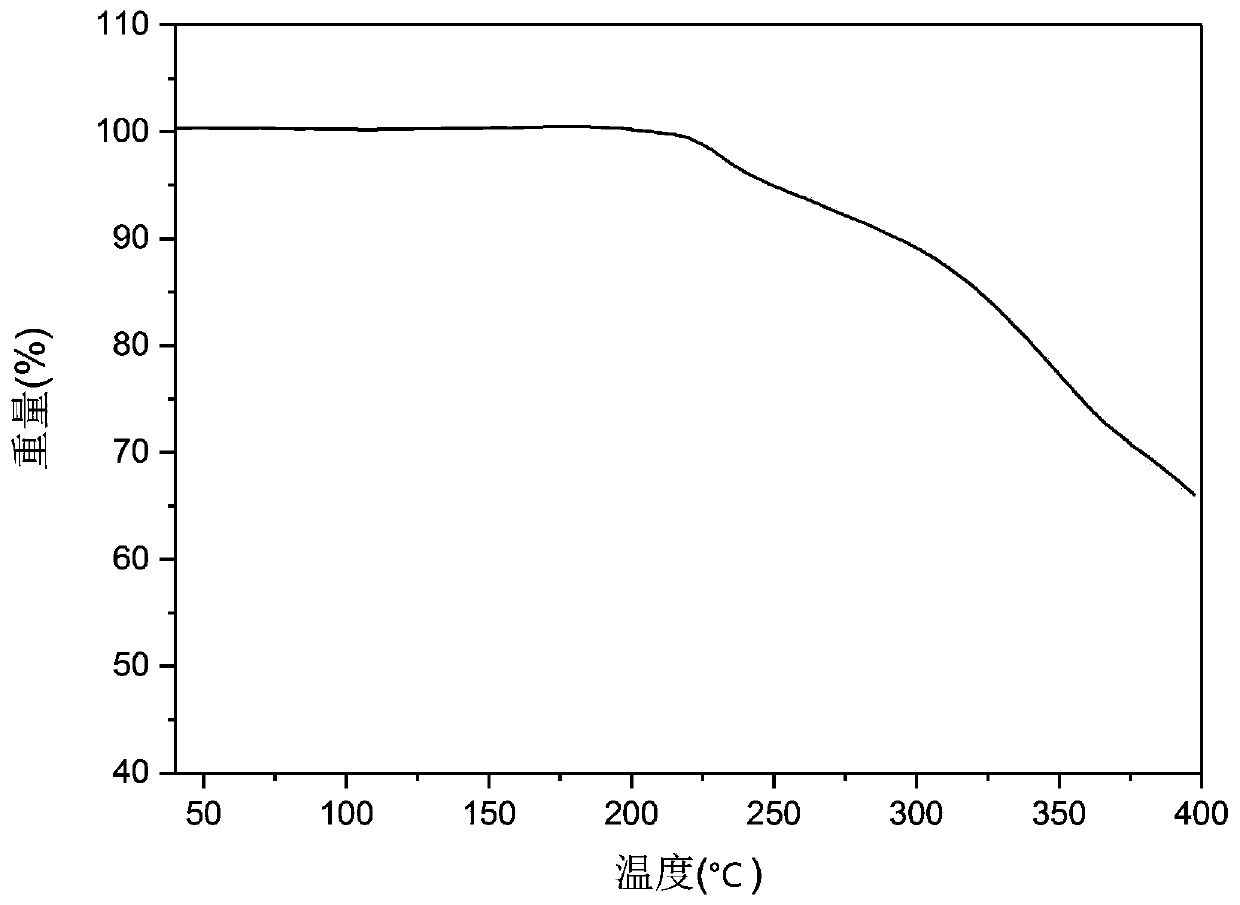
[0084] Carry out X-ray powder diffraction analysis to the A crystal form solid sample of tripterine obtained in Example 1, the analysis results are shown in figure 1 . Adopt TG209F3 type thermal gravimetric analyzer of German Netzsch Scientific Instrument Co., Ltd. to carry out thermogravimetric analysis to the A crystal form of tripteryglide produced in Example 1, the analysis results are shown in figure 2 . From figure 2 As can be seen in the figure, this crystal form A is non-hydrate or non-solvate.
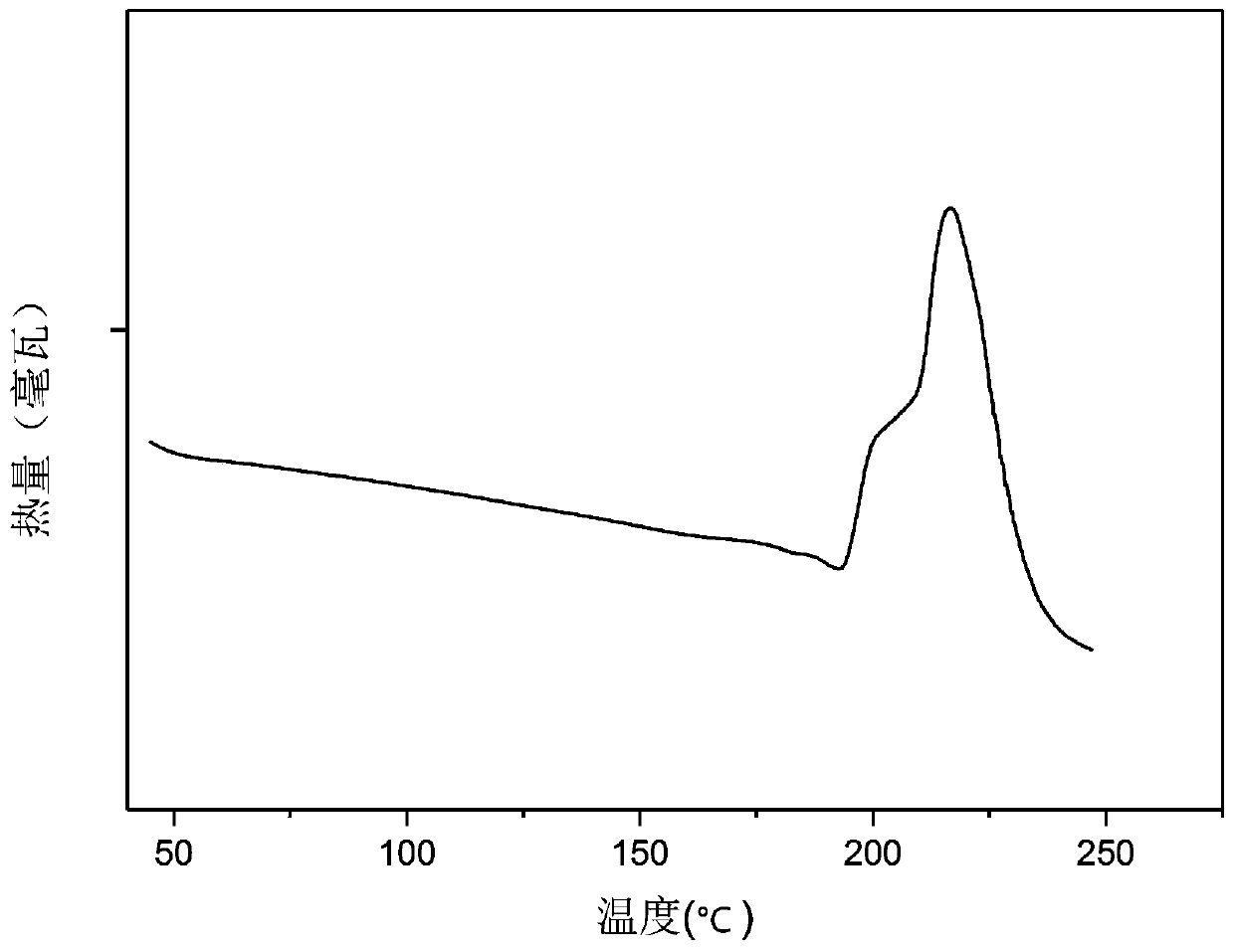
[0085] The DSC Q2000 differential scanning calorimeter of U.S. TA instrument company is used to detect that the A crystal form of tripterine produced in Example 1 is subjected to differential scannin...
Embodiment 2
[0088] Take 10 mg of tripterine as a raw material, add 5 mL of acetonitrile solvent, dissolve it by ultrasonic waves, volatilize and dry at room temperature, heat the obtained powdery solid in an oven at 150°C, and obtain tripterine A crystal form, which is red powder.
Embodiment 3
[0090] Take 50mg of tripterine raw material, add 2mL of nitromethane solvent, suspend at 25°C for 2 days, centrifuge and take powdery solid, after vacuum drying, put it into an oven at 130°C and heat it to get tripterine Form A is a red powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com