Method for producing poly(alkylene carbonate)polyol
A technology of alkylene carbonate and polyol, applied in the field of preparation of low-molecular-weight polyol, can solve the problems of tediousness, low solubility in organic solvents, difficult to control catalyst content, etc., and achieve the effect of improving activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
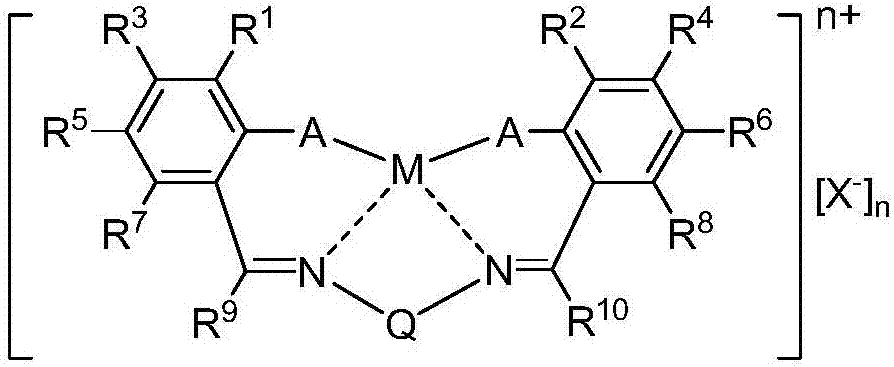
[0027] The invention provides the preparation method of low molecular weight poly(alkylene carbonate) polyol, the preparation method of low molecular weight poly(alkylene carbonate) polyol of the present invention comprises the following steps: in the presence of Salen catalyst and double metal cyanide catalyst Next, the epoxy compound, carbon dioxide, and chain transfer agent are reacted to prepare poly(alkylene carbonate) polyol.
[0028] The preparation method of low-molecular-weight poly(alkylene carbonate) polyol of the present invention uses Salen catalyst and double metal cyanide catalyst simultaneously, thereby can realize the copolymer with the high activity that Salen catalyst has and prepare high yield, and can prepare by Poly(alkylene carbonate) polyols having ether-bonded monomers within copolymers brought about by double metal cyanide catalysts.
[0029] In addition, by adjusting the weight ratio of the Salen catalyst and the double metal cyanide catalyst, the co...
preparation example 1
[0099] [Preparation Example 1] Synthesis of Catalyst (Compound 1)
[0100] Below, the simple and economical catalyst synthesis method of the present invention is listed. Compound 1 was synthesized by a known method (Bull. Korean Chem. Soc. 2009, 30, 745-748).
[0101]
[0102] Synthesis of Compound 1-2
[0103] Compound 1-1 (100mg, 0.054mmol) and AgNO 3 (37.3 mg, 0.219 mmol) was dissolved in ethanol (3 mL) and stirred overnight. The resulting AgI was filtered and removed using celite. The solvent was depressurized and removed in vacuo to obtain compound 1-2 (0.80 g, 94%) in the form of yellow solid powder.
[0104] 1 H NMR (CDCl 3 ): δ13.51 (s, 2H, OH), 8.48 (s, 2H, CH=N), 7.15 (s, 4H, m-H), 3.44 (br, 2H, cyclohexyl-CH), 3.19 (br, 32H , NCH 2 ), 2.24(s, 6H, CH 3 ), 1.57-1.52 (br, 4H, cyclohexyl-CH 2 ), 1.43-1.26 (br, 74H), 0.90-070 (br, 36H, CH 3 )ppm.
[0105] Synthesis of compound 1
[0106] Compound 1-2 (95mg, 0.061mmol) and Co(OAc) 2 (10.7 mg, 0.061 mmo...
preparation example 2
[0113] [Preparation Example 2] DMC catalyst [ZnCl] + 2 [HCo(CN) 6 ] 2- ·[CH 3 OH] (Compound 2) Synthesis
[0114] h 3 Co(CN) 6 preparation of
[0115] 5 g (15 mmol) of Potassium hexacyanocobaltate (III) was dissolved in 15 mL of distilled water, then immersed in 140 g of ion exchange resin (Dowex 5x4-200), and filtered after 3 hours. After the filtrate of the ion exchange resin was immersed in the ion exchange resin 4 times again, it was confirmed that K + Ions and H + The ions are completely exchanged. The filtered ion exchange resin can be reused after being washed with 2N sulfuric acid aqueous solution. H was separated from the filtrate by a rotary evaporator 3 Co(CN) 6 , and at P 2 o 5 The remaining moisture was removed by storing in a vacuum desiccator for 12 hours in the presence of . By titration with standard NaOH solution, it is confirmed that the metal cyanide complex salt that has been dehydrated and passed through the ion exchange resin is H 3 Co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com