Aescin A and B liposome gel and preparation method thereof
A technology of liposome gel and aescin, which is applied in liposome delivery, liquid delivery, pharmaceutical formulations, etc., can solve the problems of poor transdermal absorption effect and large skin irritation, and achieve good transdermal absorption effect , reduce the incidence, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Raw material prescription:
[0022]
[0023] Preparation Process:
[0024] 1) Preparation of liposomes: Take 6.8g of aescin A, 4.3g of aescin B, 20g of soybean lecithin and 5g of cholesterol, add 50g of absolute ethanol, stir at 60°C to dissolve, pass the resulting ethanol solution through a peristaltic pump Inject into 250g of purified water at constant temperature to 60°C at a speed of 15ml / min, stir for 30min to make a liposome suspension; then place the liposome suspension in a liposome extruder, and pass through PC filter membranes with a pore size of 200nm and 100nm were extruded 4 times each to obtain 305g of the extruded liquid; finally, the method of membrane dialysis was used to dilute the extruded liquid by adding 200g of purified water, and then dialyzed 3 times to remove the extruded liquid. The ethanol in, get aescin A, B liposome 414g;
[0025] The encapsulation efficiency and particle size of the liposomes were detected according to the literature m...
Embodiment 2
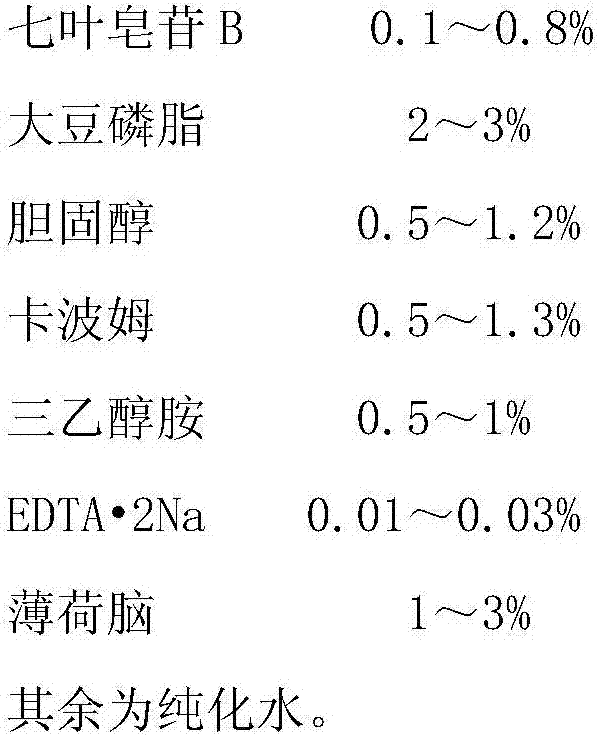
[0030] Raw material prescription:
[0031]
[0032] Preparation Process:
[0033] 1) Preparation of liposomes: Take 14g of aescin A, 4g of aescin B, 25g of soybean lecithin and 10g of cholesterol, add 100g of dehydrated ethanol, stir at 60°C to dissolve, pass the obtained ethanol solution through a peristaltic pump in 25ml The speed of / min is injected into 300g purified water with constant temperature to 60°C, and stirred for 30min to make a liposome suspension; The 200nm and 100nm PC filter membranes were extruded 4 times each to obtain 370g of the extruded liquid; finally, the method of membrane dialysis was used to dilute the extruded liquid by adding 300g of purified water, and then dialyzed 3 times to remove the extruded liquid. Ethanol, get aescin A, B liposome 505g;
[0034] The encapsulation efficiency and particle size of the liposomes were detected according to the literature method, and the encapsulation efficiency was 83%, and the average particle size was 39...
Embodiment 3
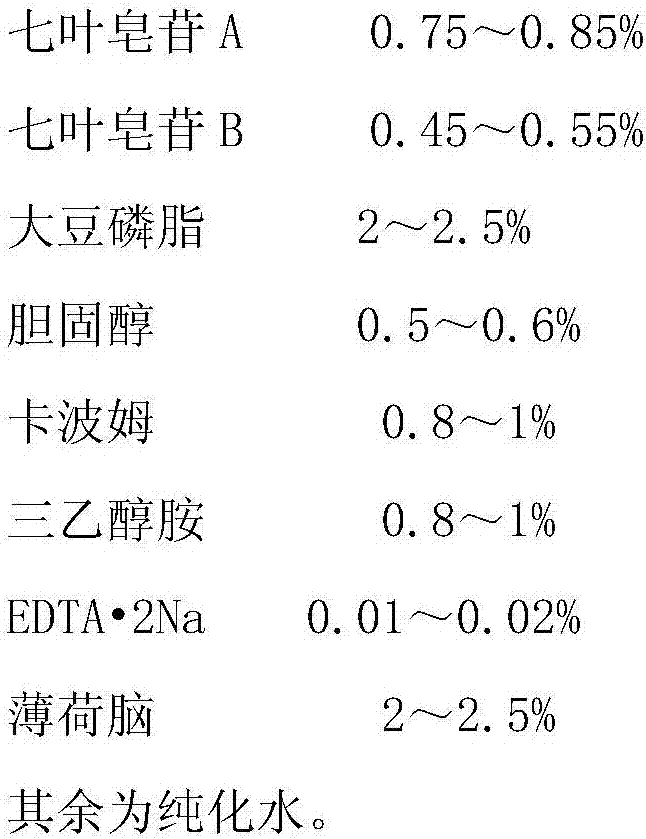
[0039] Raw material prescription:
[0040]
[0041]
[0042] Preparation Process:
[0043] 1) Preparation of liposomes: Take 3.2g of aescin A, 5.2g of aescin B, 15g of soybean lecithin and 4g of cholesterol, add 30g of absolute ethanol, stir at 60°C to dissolve, pass the resulting ethanol solution through a peristaltic pump Inject into 180g of purified water at constant temperature to 60°C at a speed of 5ml / min, stir for 30min to make a liposome suspension; then place the liposome suspension in a liposome extruder, and pass through PC filter membranes with a pore size of 200nm and 100nm were extruded 4 times each to obtain 195g of extruded liquid; finally, membrane dialysis was used to dilute the extruded liquid by adding 150g of purified water, and then dialyzed 3 times to remove the extruded liquid. The ethanol in, get aescin A, B liposome 320g;
[0044] The encapsulation efficiency and particle size of the liposomes were detected according to the literature method, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com