A construction method of photocatalytic system based on nickel nanoparticle co-catalyst
A technology of nanoparticles and co-catalysts, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Catalytic decomposition of water to hydrogen production industrialization development and other issues, to achieve high-efficiency photocatalytic hydrogen production performance, wide applicability and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
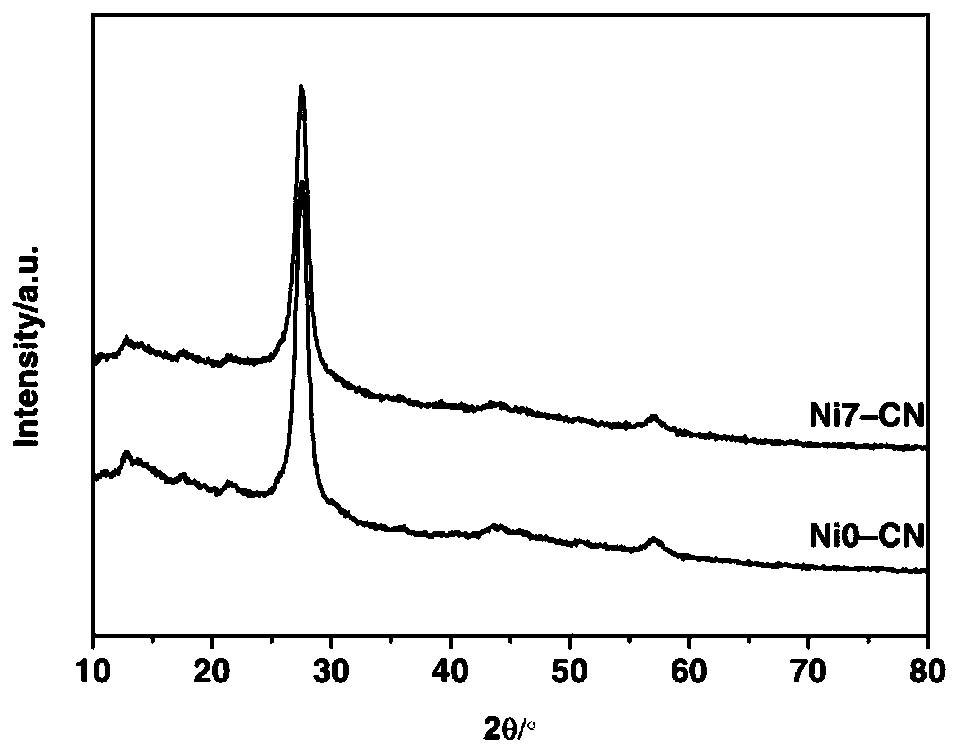
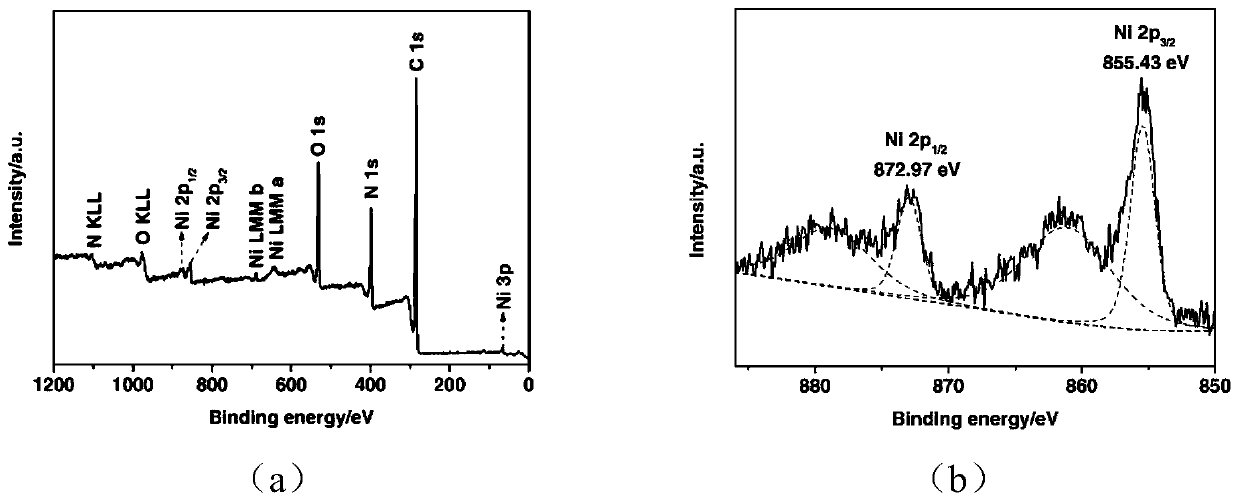
[0043] Step 1: Add 100.0mg of polyvinylpyrrolidone (PVP K30) and 20mL of ethylene glycol into a 125mL three-necked flask and dissolve completely, then place the three-necked flask in a 120°C oil bath and heat and stir until the temperature After stabilization, add 63.0 mg of NaBH to the solution 4 , The reaction solution was continuously heated and stirred for 2h to obtain a transparent solution, which was recorded as Ni0.
[0044] Step 2: Prepare g-C by roasting 10g of urea at 600°C for 3h 3 N 4 ;
[0045] Step 3: Select the prepared g-C 3 N 4 As a photocatalyst, triethanolamine was used as a sacrificial agent for hydrogen production, and NiO dispersion was added to the reaction solution for photocatalytic hydrogen production. Specific steps are as follows:
[0046] (1) Add 50.0 mg of g-C to a reactor with a volume of 250 mL 3 N 4 Photocatalyst, adding NiO dispersion liquid, and a total volume of 200mL triethanolamine content of 10vol% aqueous solution as a sacrificia...
Embodiment 2
[0050] Step 1: Add 100.0mg of polyvinylpyrrolidone (PVP K30) and 20mL of ethylene glycol into a 125mL three-necked flask and dissolve completely, then place the three-necked flask in a 120°C oil bath and heat and stir until the temperature After stabilization, add 63.0 mg of NaBH to the solution 4 , The reaction solution was continuously heated and stirred for 2h to obtain a transparent solution, which was recorded as Ni0.
[0051] Step 2: Prepare g-C by roasting 10g of urea at 600°C for 3h 3 N 4 ;
[0052] Step 3: Select the prepared g-C 3 N 4 As a photocatalyst, methanol was used as a sacrificial agent for hydrogen production, and NiO dispersion was added to the reaction solution for photocatalytic hydrogen production. Specific steps are as follows:
[0053] (1) Add 50.0 mg of g-C to a reactor with a volume of 250 mL 3 N 4 Photocatalyst, add NiO dispersion liquid, and a total volume of 200mL of methanol content of 20vol% aqueous solution as a sacrificial agent;
[0...
Embodiment 3
[0057] Step 1: Add 100.0mg of polyvinylpyrrolidone (PVP K30) and 20mL of ethylene glycol into a 125mL three-necked flask and dissolve completely, then place the three-necked flask in a 120°C oil bath and heat and stir until the temperature After stabilization, add 63.0 mg of NaBH to the solution 4 , The reaction solution was continuously heated and stirred for 2h to obtain a transparent solution, which was recorded as Ni0.
[0058] Step 2: Buy commercial P25 as TiO 2 catalyst of light;
[0059] Step 3: Select the prepared TiO 2 As a photocatalyst, methanol was used as a sacrificial agent for hydrogen production, and NiO dispersion was added to the reaction solution for photocatalytic hydrogen production. Specific steps are as follows:
[0060] (1) Add 50.0 mg of TiO to a reactor with a volume of 250 mL 2 Photocatalyst, add NiO dispersion liquid, and a total volume of 200mL of methanol content of 20vol% aqueous solution as a sacrificial agent;
[0061] (2) Nitrogen was pu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com