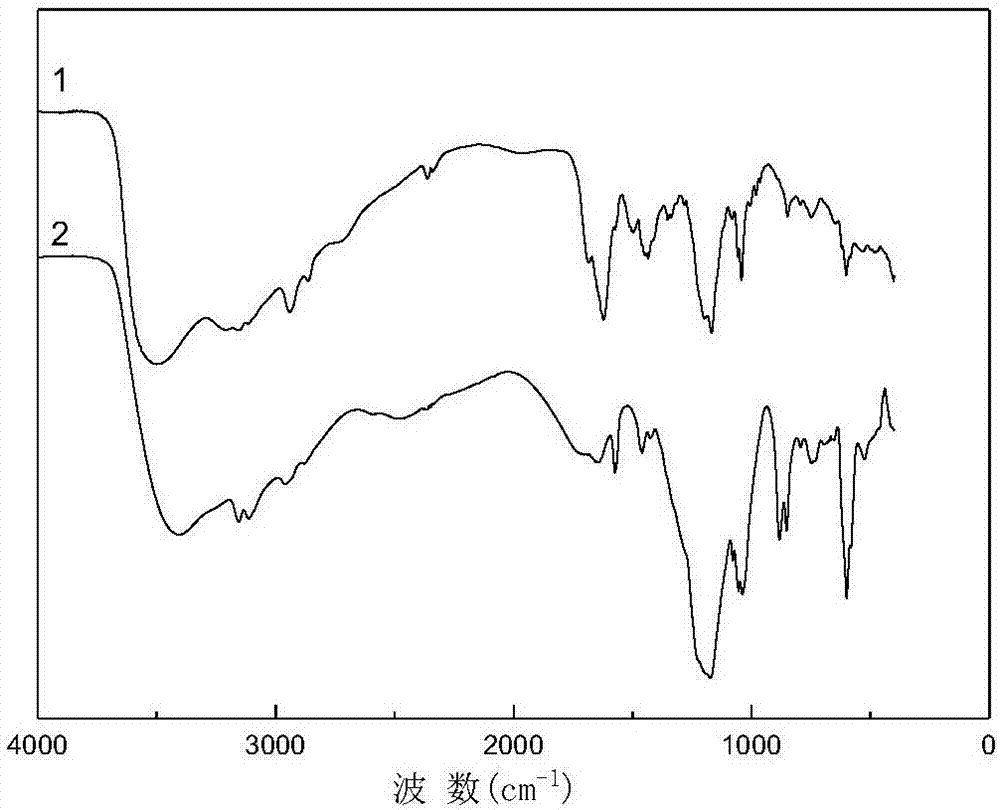
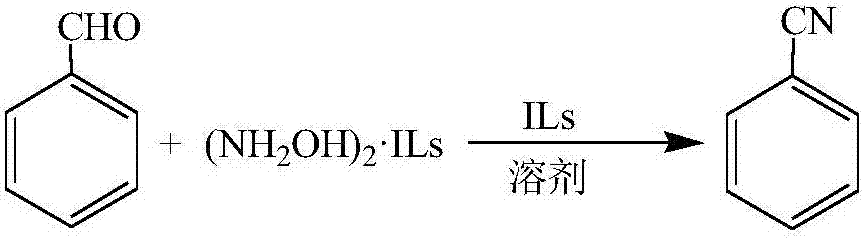Method for one-step synthesis of benzonitrile from benzaldehyde and ionic liquid type hydroxylammonium salt
An ionic liquid, benzaldehyde technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid nitrile, etc., can solve the problems of unfriendly environment, complex production process of benzonitrile, equipment corrosion, etc. Friendly, achieves clean synthesis, and does not corrode equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add benzaldehyde (3.6mmol), N,N,N-trimethyl-N-sulfobutylammonium bisulfate ionic liquid type hydroxylamine salt (5.4mmol) and zinc chloride catalyst (0.144mmol) into a 100mL three-necked flask , add 10mL of toluene and 2mL of N,N,N-trimethyl-N-sulfobutyl ammonium bisulfate ionic liquid, stir, reflux and condense, react at normal pressure and 100°C for 8h, then stop the reaction. The reaction solution was cooled to room temperature, and the supernatant was directly analyzed on a gas chromatograph. The result of the reaction was that the conversion rate of benzaldehyde was 87.9%, and the yield of benzonitrile was 87.1%.
Embodiment 2
[0035] The other steps are the same as in Example 1, except that the added N,N,N-trimethyl-N-sulfobutylammonium bisulfate ionic liquid type hydroxylamine salt is 4.32mmol, and the reaction result is that the conversion rate of benzaldehyde is 68.1 %, the yield of benzonitrile is 65.9%.
Embodiment 3
[0037] Other steps are the same as in Example 1, except that the added N,N,N-trimethyl-N-sulfobutyl ammonium bisulfate ionic liquid type hydroxylamine salt is 4.68mmol, and the reaction result is that the conversion rate of benzaldehyde is 73.6 %, the yield of benzonitrile is 70.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com