Kit for detecting keratoprotein 19 fragment and carcino-embryonic antigen contents in sample based on luminous quantum dot nanometer microemulsion probe
A technology for detecting samples and luminescent quanta, applied in the field of immunodetection, can solve the problems of difficult storage, easy to be photobleached, weak fluorescent signal, etc., and achieve the effect of short reaction time, overcoming the problem of precipitation, and strong fluorescent signal.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] A kit for detecting the content of keratinin 19 fragments and carcinoembryonic antigen in a sample based on a luminescent quantum dot nanomicroemulsion probe, including an immunochromatographic test strip and a functional luminescent quantum dot nanomicroemulsion probe. Among them, the functional luminescent quantum dot nano-microemulsion probes include three types, one is a functional luminescent quantum dot nano-microemulsion probe coupled to carcinoembryonic antigen monoclonal antibody S001, and the other is a monoclonal antibody coupled to keratin 19 fragment. A functional luminescent quantum dot nano-microemulsion probe for cloning antibodies, and a functional luminescent quantum dot nano-microemulsion probe coupled to rabbit IgG.
[0050] 1. Preparation of functional quantum dot nano-microemulsion probes, the method is as follows:
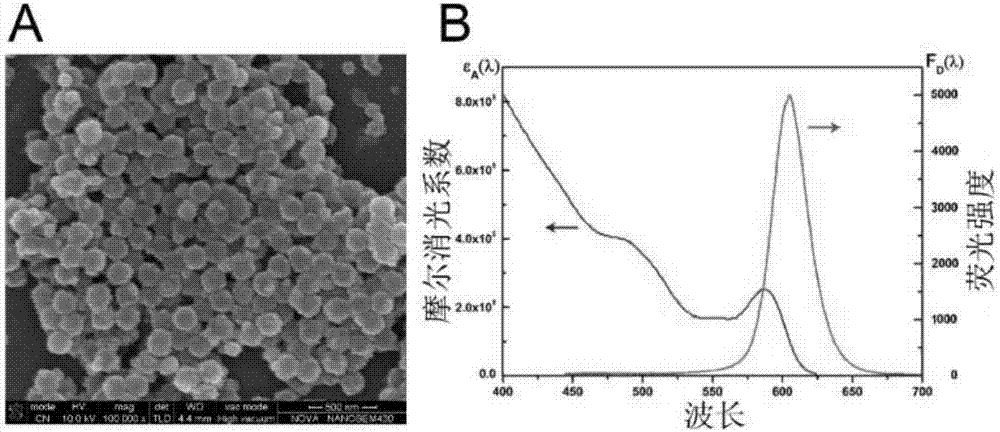
[0051] 1. Pretreatment of latex spheres: centrifuge 100µL nano latex spheres (particle size 205±3nm, mass concentration 100mg / mL, sol...
Embodiment 2
[0058] A kit for detecting the content of keratinin 19 fragments and carcinoembryonic antigen in a sample based on a luminescent quantum dot nanomicroemulsion probe, including an immunochromatographic test strip and a functional quantum dot nanomicroemulsion probe.
[0059] 1. The preparation of functional quantum dot nano-microemulsion probes is the same as in Example 1.
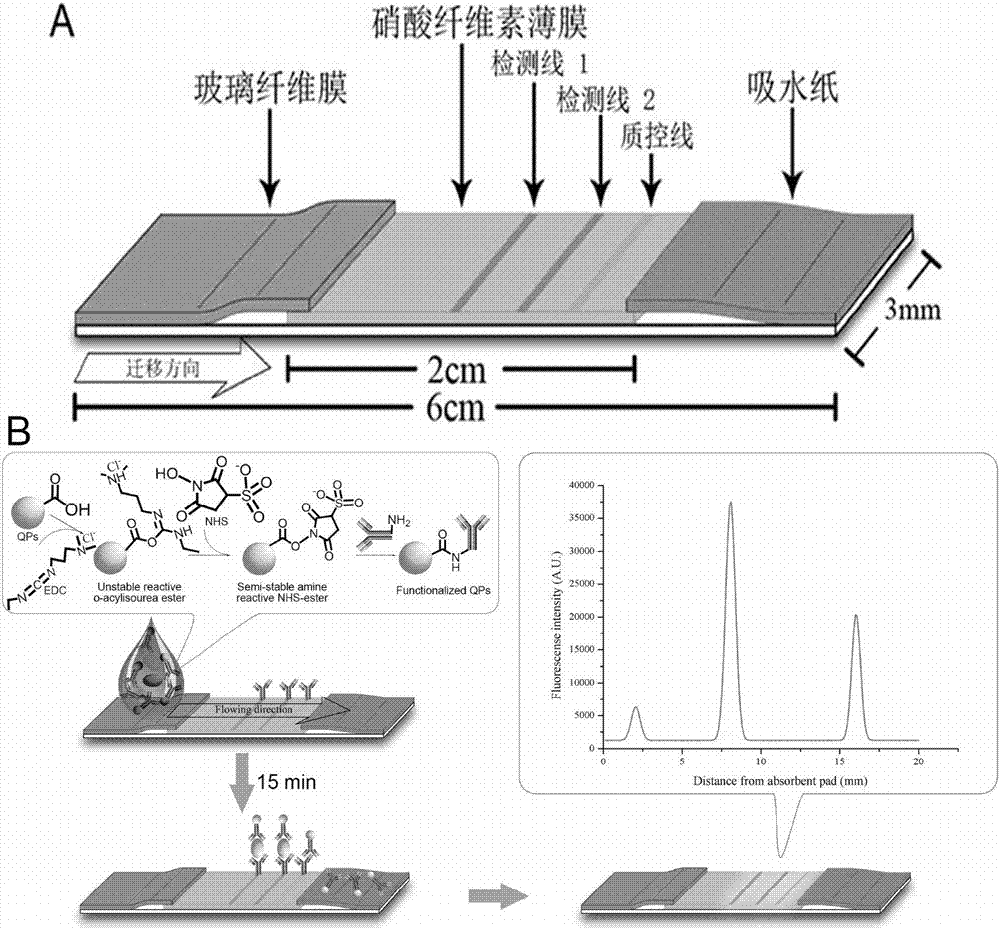
[0060] 2. The structure of the immunochromatographic test strip is as follows: figure 1 Shown, the preparation method of immunochromatographic test strip is as follows:
[0061] 1. Pretreatment of the sample pad: cut the glass fiber membrane into strips of 1.2cm×30cm, then soak the strip glass fiber membrane in the sample pad treatment solution, and soak for two hours at room temperature. After absorbing the liquid with absorbent paper, dry it overnight at 37°C, and store the dried sample pad in a dry and cool place until use.
[0062] 2. Treatment of the nitrocellulose film: Cut the nitrocellulose film i...
Embodiment 3
[0071] Methodological verification of the present invention is carried out verification to this analytical method according to routine manufacture and verification procedure in this area, and the result is as follows:
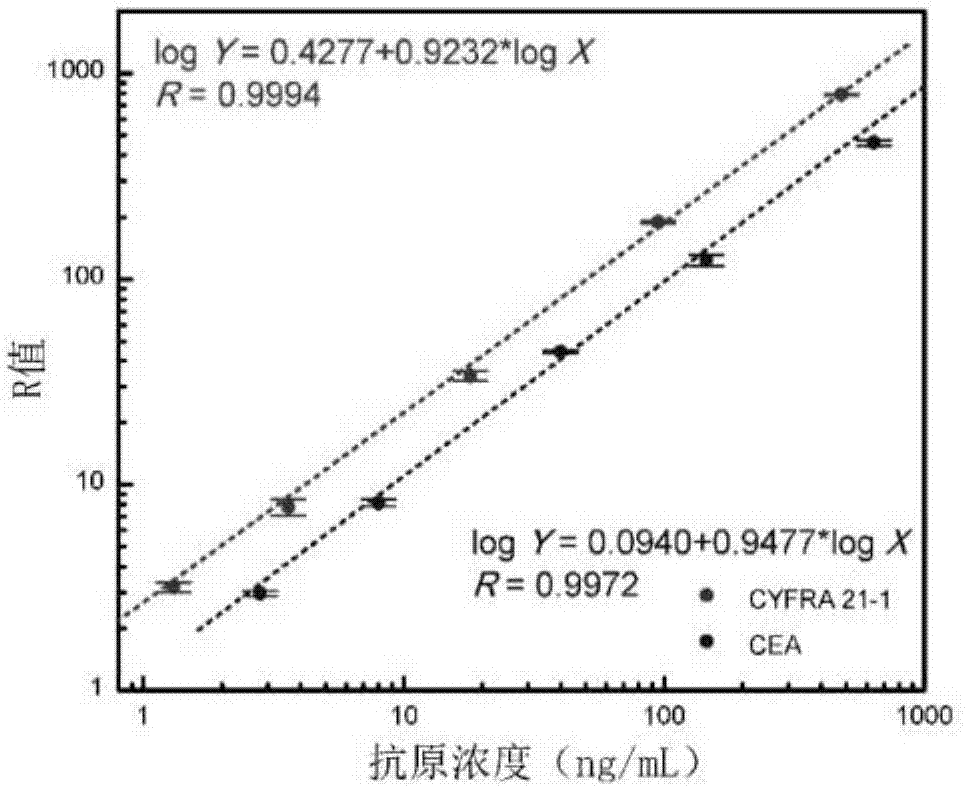
[0072] 1. Analytical sensitivity and linear range: Measure 8 times with the zero reference standard as the sample, and calculate its R average value and standard deviation. The concentration calculated by substituting the fluorescence value obtained by adding the average value of R at this point plus 2 times the standard deviation into the standard curve equation is its sensitivity. It has been determined that the sensitivity of this reagent to CYFRA 21-1 analysis is 0.16 ng / mL, and the sensitivity to CEA analysis is 0.16 ng / mL. 0.35 ng / mL. The antigens were diluted into different concentrations for determination, and the linear ranges of the measured standard curves were CYFRA 21-1: 1.3~500ng / mL; CEA: 1.2~1000ng / mL.
[0073] 2. Precision: measure the self-mad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com