Photocatalytic hydrogen production catalyst and preparation method thereof
A catalyst and photocatalysis technology, applied in the direction of catalyst activation/preparation, physical/chemical process catalyst, chemical instruments and methods, etc., to achieve the effects of good stability, simple preparation method and good photocatalytic hydrogen production activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1) 5.3126g niobium pentoxide and 3.69g glucose (n 五氧化二铌 :n 葡萄糖 =1:1) Grind in a mortar for 30min, then add the ground mixture into 70ml of 14M potassium hydroxide solution and stir for 1h, then transfer the mixed solution to a 100ml polytetrafluoroethylene reactor, and conduct a hydrothermal reaction at 160°C 12h, then cooled to room temperature. The final product was washed with alcohol and water, and dried at 60°C for 24 hours to obtain carbon-doped potassium niobate (C-KNbO 3 );
[0021] 2) Weigh 0.5g of carbon-doped potassium niobate, 0.0034g of sodium molybdate and 0.0068g of thiourea respectively, disperse them in 70ml of deionized water, stir for 30min, then ultrasonicate for 30min, then transfer the mixture to 100ml polytetrafluoroethylene In a vinyl fluoride reactor, hydrothermally react at 200°C for 24h, and then cool to room temperature. The final product was washed with alcohol and water, and dried at 60°C for 24 hours to obtain MoS with a molar ratio of...
Embodiment 2
[0024] 1) with the step of (1) in embodiment 1;
[0025] 2) Weigh 0.5g of carbon-doped potassium niobate, 0.0014g of sodium molybdate and 0.0028g of thiourea respectively, disperse them in 70ml of deionized water, first stir for 30min, then ultrasonicate for 30min, then transfer the mixture to 100ml polytetrafluoroethylene In a vinyl fluoride reactor, hydrothermally react at 200°C for 24h, and then cool to room temperature. The final product was washed with alcohol and water, and dried at 60°C for 24 hours to obtain MoS with a molar ratio of 0.002 2 / C-KNbO 3 .
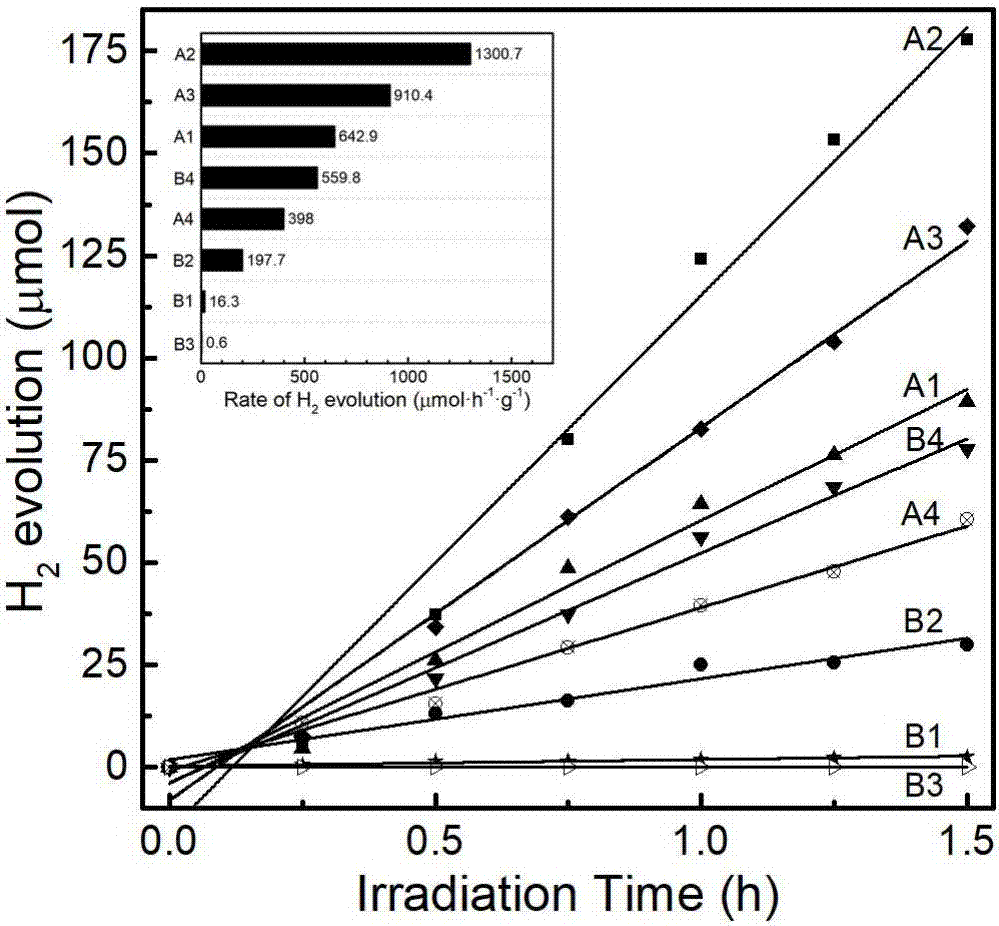
[0026] Photocatalytic hydrogen production activity see figure 1 in A2.
Embodiment 3
[0028] 1) with the step of (1) in embodiment 1;
[0029] 2) Weigh 0.5g of carbon-doped potassium niobate, 0.0007g of sodium molybdate and 0.0014g of thiourea respectively, disperse them in 70ml of deionized water, stir for 30min, then ultrasonicate for 30min, then transfer the mixture to 100ml of polytetrafluoroethylene In a vinyl fluoride reactor, hydrothermally react at 200°C for 24h, and then cool to room temperature. The final product was washed with alcohol and water, and dried at 60°C for 24 hours to obtain MoS with a molar ratio of 0.001 2 / C-KNbO 3 .
[0030] Photocatalytic hydrogen production activity see figure 1 in A3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com