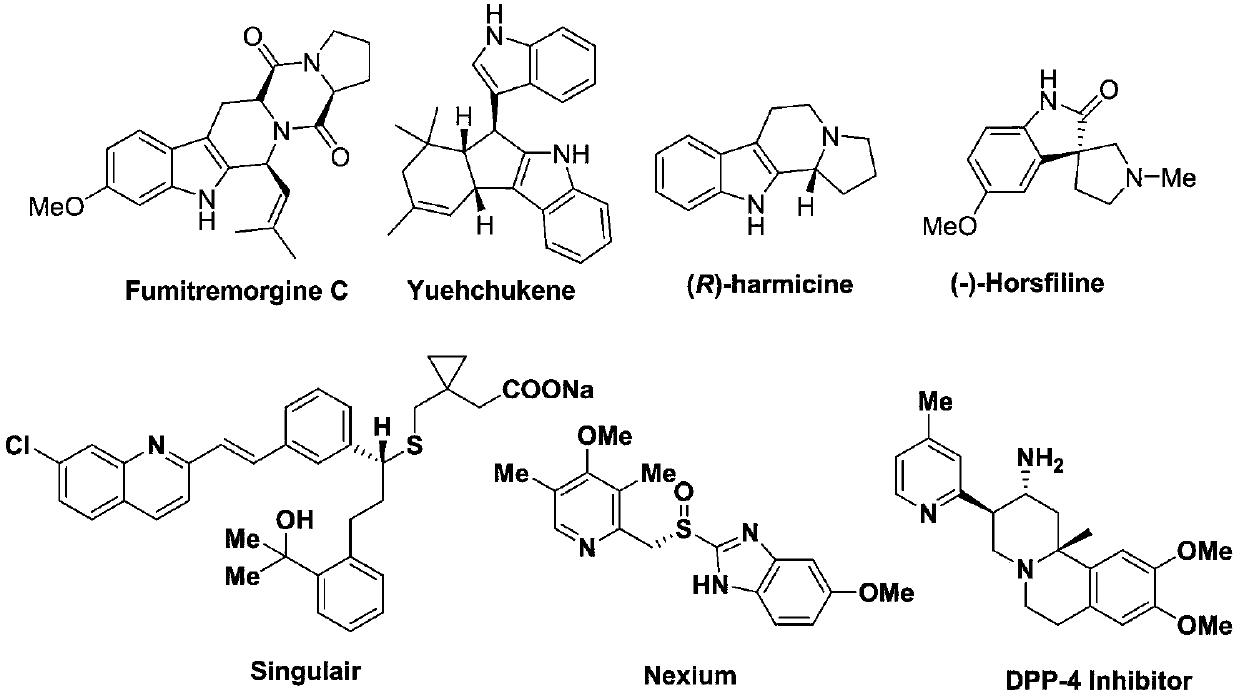
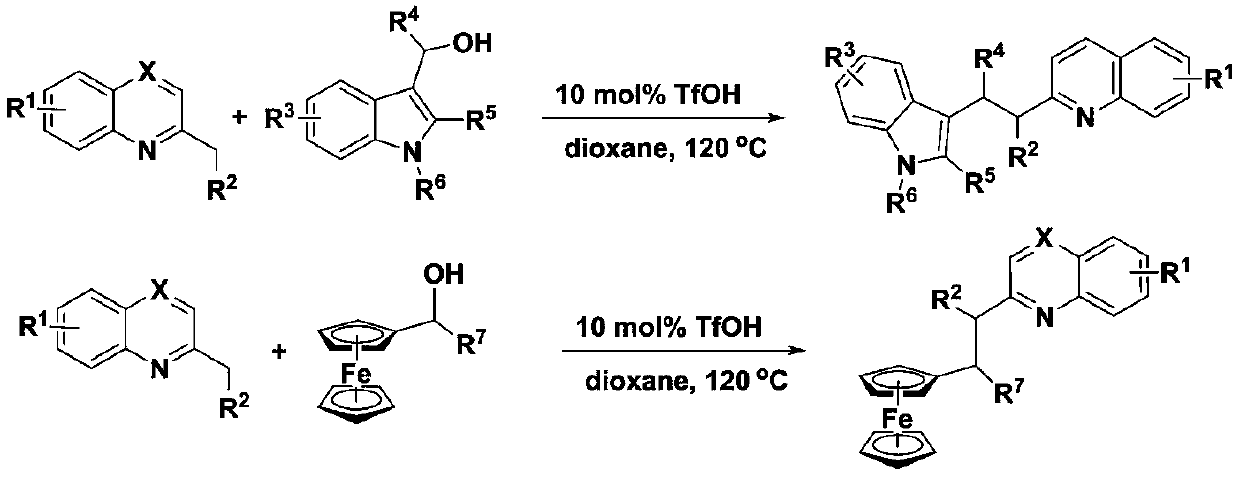
A method for synthesizing indole substituted or ferrocene substituted azaarenes
A technology of azaaromatics and ferrocene, applied in the field of chemical synthesis, can solve the problems of high cost, limited application, large pollution, etc., and achieve the effects of convenient separation, simple and practical operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: optimization of conditions
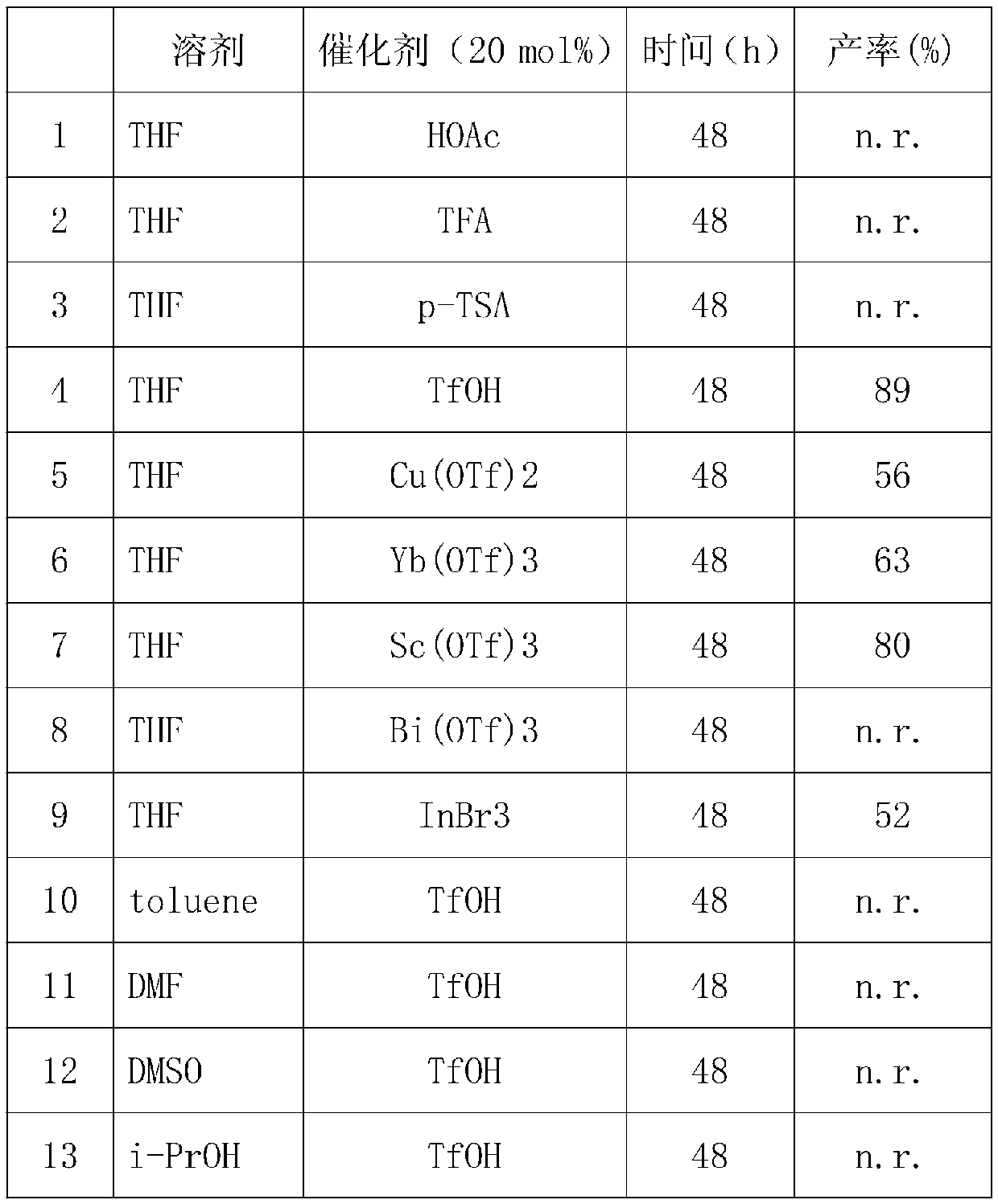
[0034] Take 0.2mmol of 2-methylquinoline compounds, 0.1mmol of indole alcohol or ferrocenol and 0.01mmol of TfOH in a sealed tube, add 2mL of solvent, stir at 120°C for reaction, and determine the reaction by TLC. After the reaction is completed, cool to room temperature, rotary evaporate, and chromatographic column separation.
[0035] Under the same reaction conditions, the solvents used are different, and the reaction yields are also different, and the specific yields are as follows:
[0036]
[0037]
[0038] Note: Yields are isolated yields.
Embodiment 2
[0039] Embodiment 2: the method that synthetic indole replaces or ferrocene replaces azaarene:
[0040]
Embodiment 3
[0042]
[0043] The product that present embodiment makes carries out nuclear magnetic resonance analysis:
[0044] Isolated yield: 93%, 1H NMR (500MHz, DMSO) δ11.06(s, 1H), 8.01(d, J=8.4Hz, 1H), 7.85(d, J=8.4Hz, 1H), 7.81(d, J =8.0Hz,1H),7.74(d,J=8.0Hz,1H),7.68(t,J=7.6Hz,1H),7.49(t,J=7.4Hz,1H),7.40-7.32(m,7H ),7.30(d,J=8.0Hz,1H),7.22(t,J=7.6Hz,2H),7.11(t,J=7.3Hz,1H),7.06(t,J=7.5Hz,1H), 7.02(d, J=8.4Hz, 1H), 6.97(t, J=7.5Hz, 1H), 5.25–5.19(m, 1H), 3.98–3.88(m, 2H).
[0045] 13C NMR (125MHz, DMSO) δ161.0, 147.3, 144.9, 136.3, 135.7, 135.5, 132.9, 129.3, 128.6, 128.4, 128.3, 128.2, 127.7, 127.6, 127.4, 127.0, 126.4, 125.1.7, 125 ,118.8,113.1,111.5,42.5,41.1.
[0046] HRMS(ESI):calcd.for C31H24N2[M+H]+:425.2018,found:425.2020
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com