Multiplex real-time fluorescent quantitative PCR (Polymerase Chain Reaction) rapid diagnosis kit for five porcine diarrhea viruses and application
A real-time fluorescence quantitative and rapid diagnosis technology, which is applied in the direction of recombinant DNA technology, microbial measurement/inspection, and resistance to vector-borne diseases, can solve the problems of cumbersome operation, time-consuming and labor-intensive, low sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1, plasmid standard product preparation
[0075] Step 1: Primer Synthesis
[0076] The 5 kinds of viral primer sequences designed in the present invention (see Table 1) were synthesized in Shanghai Sangon Biology Technology Service Company, China, and the synthetic amount was 3 OD per primer.
[0077] Step 2: Total viral DNA / RNA extraction
[0078] Put 100uL of each positive sample containing TGEV, GAR, GCR, PEDV and PCV2 into a 1.5ml centrifuge tube, and use the Viral RNA / DNA Extraction Kit MiniBEST Viral RNA / DNA Extraction KitVer.4.0 (TAKARA) according to the product instruction manual Extract to obtain viral RNA / DNA.
[0079] Step 3: cDNA synthesis by reverse transcription
[0080] Reverse transcription reaction: Add 2 μl of the total RNA / DNA template prepared in the previous step to an RNase-free 0.2ml PCR tube, and perform RT according to the operating instructions of TaKaRa One Step RNA PCR Kit (AMV) (Code No.RR024A). -PCR reaction, after the revers...
Embodiment 2
[0085] Embodiment 2, the present invention detects five kinds of virus-specific experiments
[0086] Step 1: Primer Synthesis
[0087] The 5 kinds of viral primer sequences designed in the present invention (see Table 1) were synthesized in Shanghai Sangon Biology Technology Service Company, China, and the synthetic amount was 3 OD per primer.
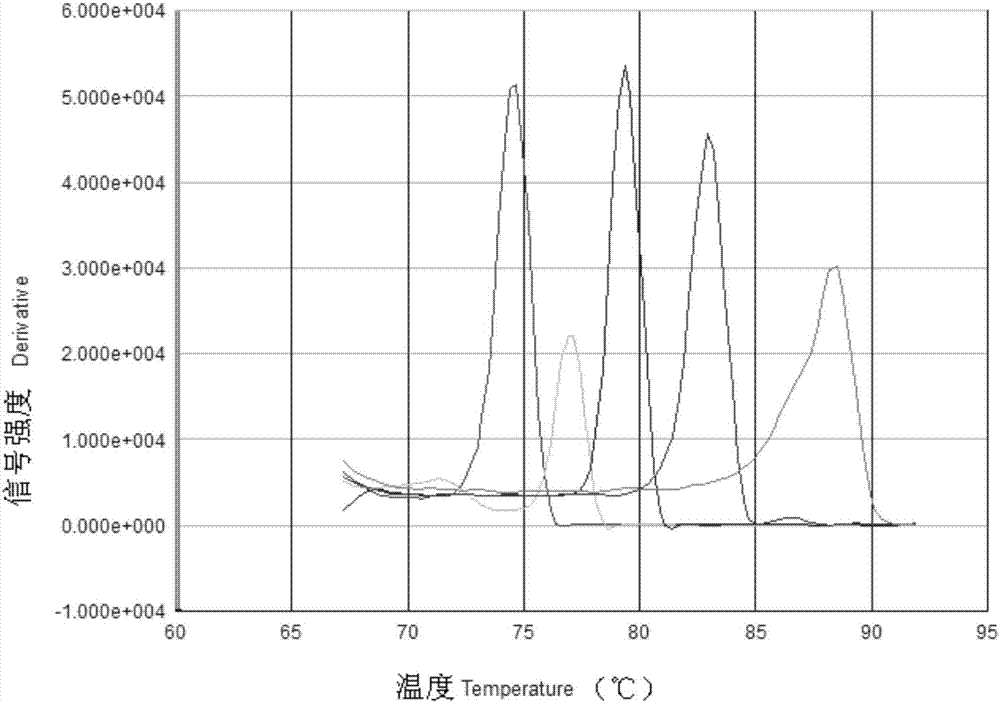
[0088] Step 2: Specific detection of a single virus in a multiplex system
[0089] Prepare 5 identical reaction solutions according to the EvaGreen multiplex real-time fluorescent quantitative PCR reaction system (i.e., 15 μL of Master Mix, 20×EvaGreen 1 μl, 10 μM TGEV, GAR, GCR, PEDV and PCV2 upstream and downstream primers are 0.5 μL, 0.5 μL, 0.15μL, 0.15μL, 0.4μL), respectively add 1μl of 1.0×10 6 Copies / μl of plasmid standards for TGEV, GAR, GCR, PEDV, and PCV2, plus ddH 2 O to a total volume of the reaction system of 20 μl; the reaction was carried out on an ABI 7300 fluorescence quantification instrument, and the reaction para...
Embodiment 3
[0091] Embodiment 3, the present invention detects five kinds of virus stability experiments
[0092] Step 1: Primer Synthesis
[0093] With the first step in embodiment 1.
[0094] Perform the following operations on TGEV, GAR, GCR, PEDV and PCV2 respectively:
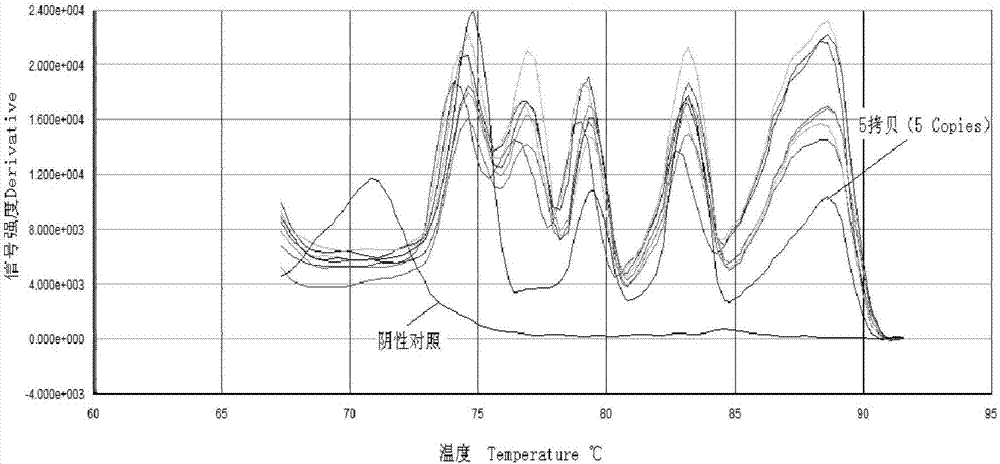
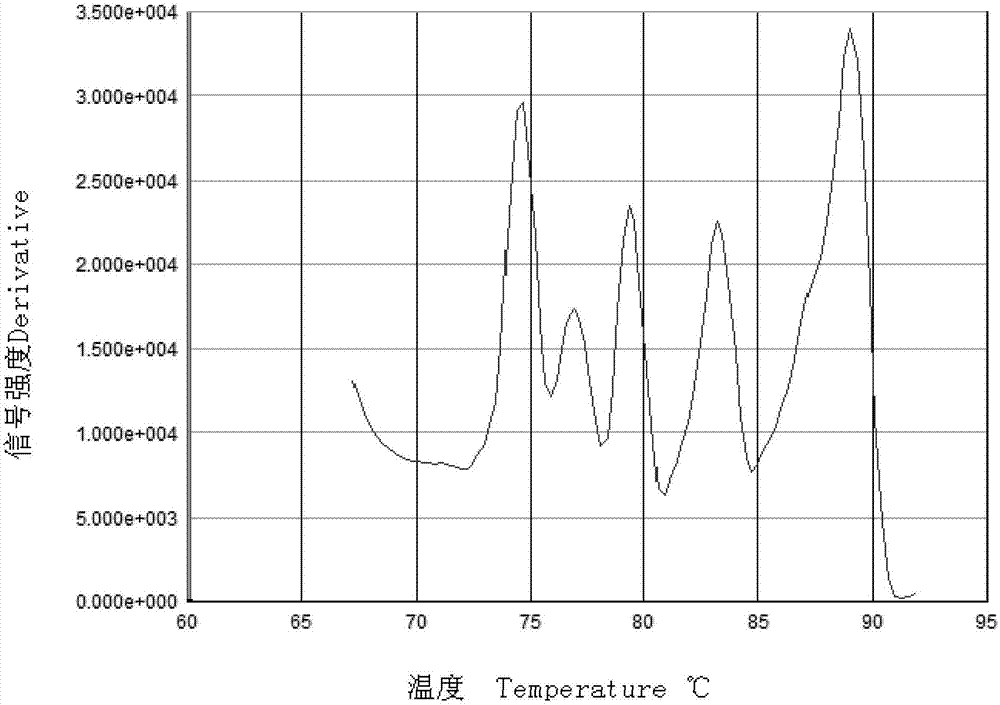
[0095] Take 6 copies of each virus 1.0×10 6 Copies / μl of plasmid standard and 2 negative controls (DEPC-H 2 0), repeated experiments were carried out within and between batches, respectively. The intra-batch repeat test is to repeat the 3 samples in the same real-time fluorescence three times; the inter-batch repeat test is to carry out the real-time fluorescent quantitative PCR test in 3 different time experiments (interval 3 days). The real-time fluorescent quantitative PCR reaction system and reaction procedure are shown in the second step of the above-mentioned embodiment 2.
[0096] The CV values of the inter-batch and intra-batch repeated tests of the five viruses are all less than 4%, and the invention h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com