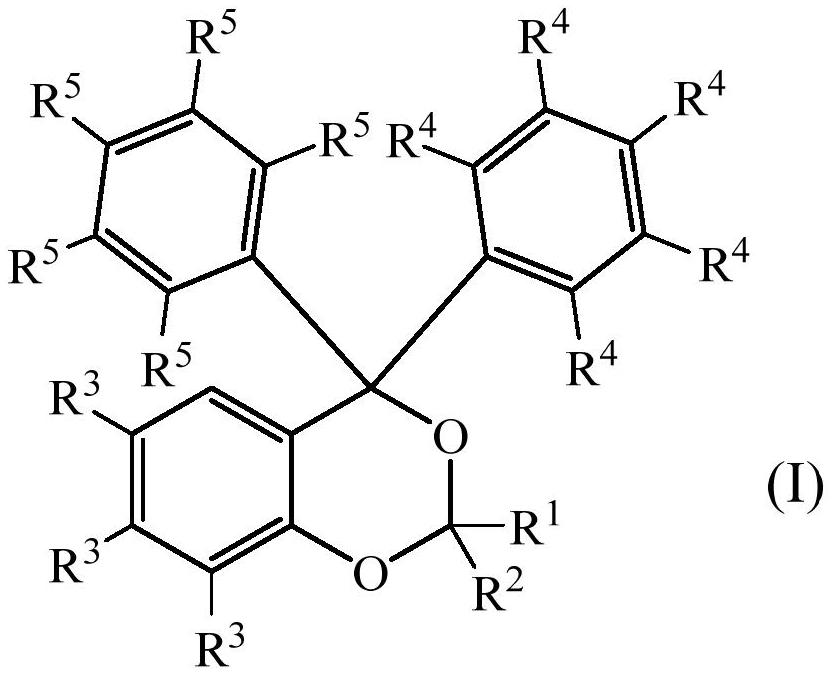
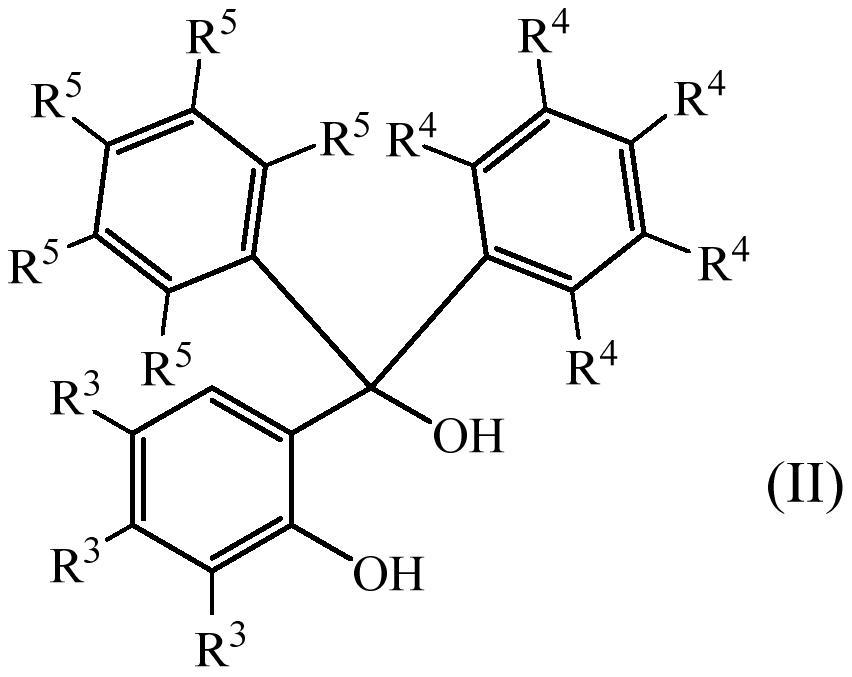
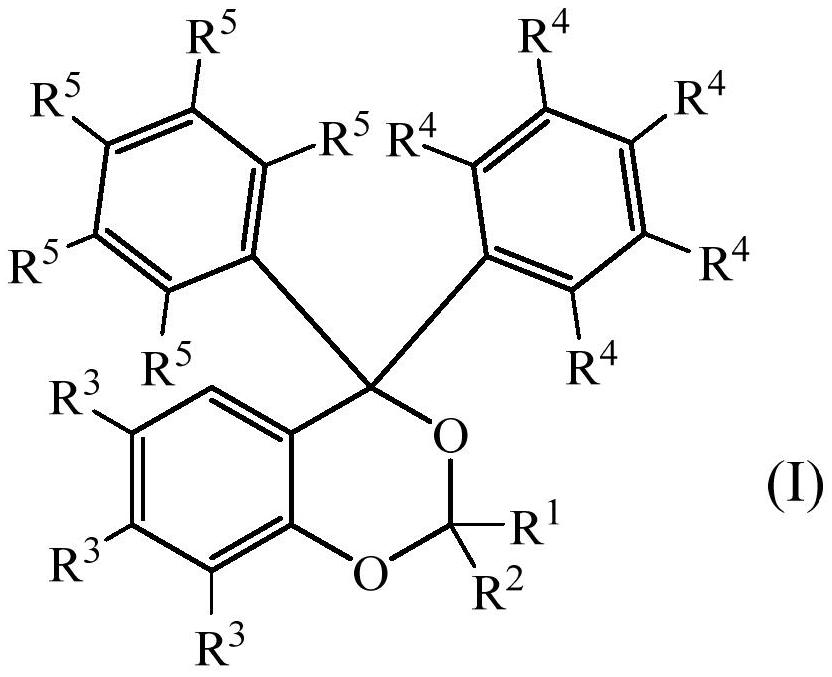
Photolabile acetal and ketal compounds for controlled release of reactive volatile carbonyl compounds
A technology for releasing active compounds, applied in softening compositions, detergent compositions, fragrances, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Preparation of non-commercial diols of formula (II) according to the invention
[0078] a) Preparation of 2-(bis(3-(dimethylamino)phenyl)(hydroxyl)methyl)phenol
[0079] Phenyl lithium (2M in cyclohexane, 9.86 mL, 19.7 mmol) was added to a solution of methyl salicylate (3.00 g, 19.7 mmol) in tetrahydrofuran (THF, 20 mL) at -78 °C over 15 minutes . After stirring at -78°C for 5 minutes, the reaction mixture was transferred to a solution of 3-bromo-N,N-dimethylaniline (7.89 g, 39.4 mmol) and magnesium turnings (1.15 g, 47.3 mmol) in THF over 20 minutes. (approximately 110 mL) of the Grignard reagent prepared in (approximately 110 mL) was kept at 0 °C during the addition. The reaction mixture was warmed to room temperature. After stirring at room temperature for 24 hours, the reaction mixture was decanted and quenched with saturated ammonium chloride solution (about 200 mL). Extracted with ethyl acetate, dried (Na 2 SO 4 ) and concentrated to give 7.97 g of crude p...
Embodiment 2
[0103] Preparation of photosensitive acetal or ketal of formula (I) according to the present invention
[0104] a) (±)-3,3'-(2-(9-decenyl)-4H-benzo[d][1,3]dioxane-4,4-diyl)bis(N, Synthesis of N-dimethylaniline)
[0105] 2-(bis(3-(dimethylamino)phenyl)(hydroxyl)methyl)phenol (0.50g, 1.4mmol) and 10-undecenal (0.46g, 2.8mmol) were mixed at 140°C The mixture was stirred for 2 hours to obtain a crude reaction product. Column chromatography (SiO 2 , heptane / ethyl acetate 3:1) and bulb-to-bulb distillation (110° C., 0.2 mbar) to remove residual 10-undecenal to yield 0.61 g (86%) of the title compound.
[0106] 1 H-NMR: 7.17(t,1H),7.14-7.09(m,1H),7.08(t,1H),6.91-6.84(m,2H),6.81-6.74(m,3H),6.72-6.66(m ,2H),6.64-6.57(m,1H),6.56-6.51(m,1H),5.86-5.75(m,1H),5.10-5.05(m,1H),5.02-4.89(m,2H),2.87 (s,6H),2.83(s,6H),2.08-1.99(m,2H),1.92-1.77(m,2H),1.58-1.41(m,2H),1.41-1.15(m,10H).
[0107] 13 C-NMR:152.38,150.31,150.14,147.02,145.17,139.21,129.83,128.29,128.15,127.94,126.21,119.75,11...
Embodiment 3
[0141] All R in formula (I) 4 and R 5 Preparation of Photosensitive Acetal or Ketal Compounds Both Are Hydrogen Atoms
[0142] a) Synthesis of 2-(hydroxydiphenylmethyl)phenol
[0143] Bromobenzene (27.70 g, 176 mmol) was weighed into a dropping funnel, and to a suspension of magnesium turnings (5.10 g, 210 mmol) in diethyl ether (10 mL) were added about 50 drops of pure compound and a few crystals of iodine. Diethyl ether (50 mL) was then added to the residual bromobenzene and the solution was added dropwise over 30 minutes. The reaction mixture was heated to reflux for 1 hour. After cooling to room temperature, salicylic acid (2.89 g, 21 mmol) in diethyl ether (50 mL) was added dropwise over 30 minutes. The reaction mixture was stirred overnight at room temperature, then poured into aqueous HCl (10%, 100 mL) and ice (100 g). Extracted with diethyl ether (2 x 100 mL), using aqueous NaOH (10%, 100 mL) and ice (50 mL), saturated NaHCO 3 aqueous solution (2x 50mL), satur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com