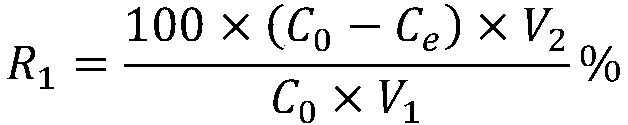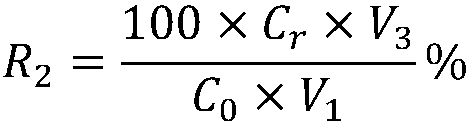Acid-resistant chelating resin combinational adjusting and controlling method for quality-divided recycling complex heavy metal
A chelating resin, heavy metal technology, applied in chemical instruments and methods, process efficiency improvement, water/sludge/sewage treatment, etc. Treatment costs and environmental risks, zero emissions and resource utilization, efficient concentration and purification effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
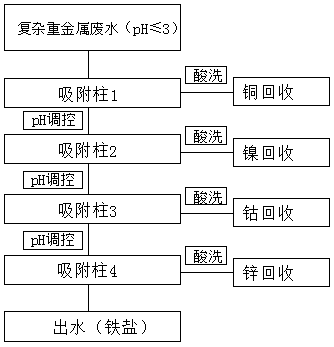
[0043] First, the wastewater containing copper, nickel and iron (both 50mg / L) with an initial pH value of 0.5 is pumped into the adsorption column filled with 5mL resin A The adsorption temperature is controlled at 20°C, the adsorption flow rate is 8BV / h, the effluent is collected and the pH value is adjusted to 1.2, and then pumped into the adsorption column filled with 5mL resin A The adsorption temperature was controlled at 20°C, the adsorption flow rate was 4BV / h, and the effluent was collected again. The chelating resin after selective adsorption of copper and nickel was desorbed and regenerated with 20% sulfuric acid and 10% hydrochloric acid respectively. The regeneration temperature was 20°C and the flow rate was 4BV / h. The desorbed and regenerated chelating resin is washed with water to neutrality and can be reused.
Embodiment 2
[0045] First, the wastewater containing copper, cobalt and iron (both 50mg / L) with an initial pH value of 0.8 is pumped into the adsorption column filled with 5mL resin A The adsorption temperature is controlled at 30°C, the adsorption flow rate is 6BV / h, the effluent is collected and the pH value is adjusted to 1.9, and then pumped into the adsorption column filled with 5mL resin B The adsorption temperature was controlled at 30°C, the adsorption flow rate was 5BV / h, and the effluent was collected again. The chelating resin after selective adsorption of copper and cobalt was desorbed and regenerated with 20% nitric acid and 10% hydrochloric acid respectively. The regeneration temperature was 40°C and the flow rate was 10BV / h. The desorbed and regenerated chelating resin is washed with water to neutrality and can be reused.
Embodiment 3
[0047] First, the wastewater containing copper, zinc and iron (both 50 mg / L) with an initial pH value of 1.1 is pumped into an adsorption column filled with 5 mL of resin A The adsorption temperature is controlled at 25°C, the adsorption flow rate is 3BV / h, the effluent is collected and the pH value is adjusted to 2.4, and then pumped into the adsorption column filled with 5mL resin B The adsorption temperature was controlled at 25°C, the adsorption flow rate was 3BV / h, and the effluent was collected again. The chelating resin after selective adsorption of copper and zinc was desorbed and regenerated with 10% sulfuric acid and 5% hydrochloric acid respectively. The regeneration temperature was 15°C and the flow rate was 1BV / h. The desorbed and regenerated chelating resin is washed with water to neutrality and can be reused.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption temperature | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com