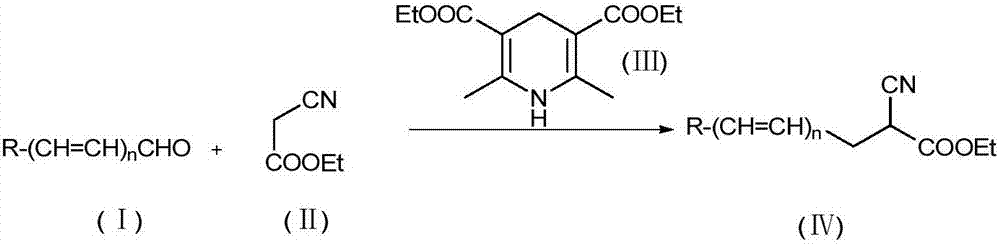
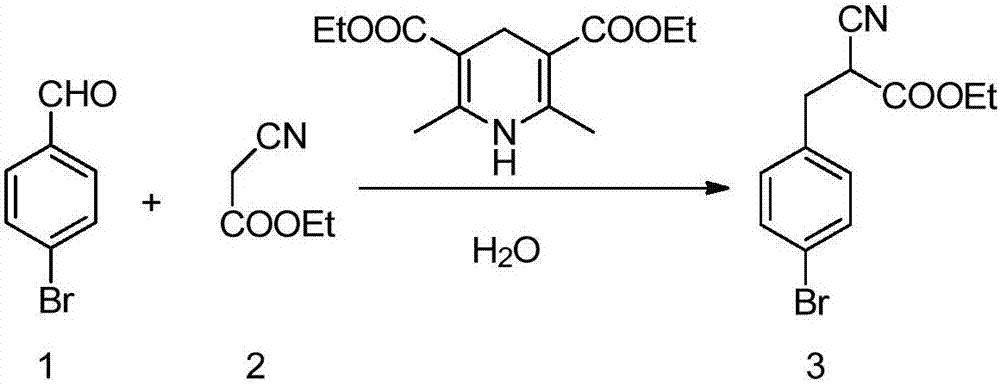
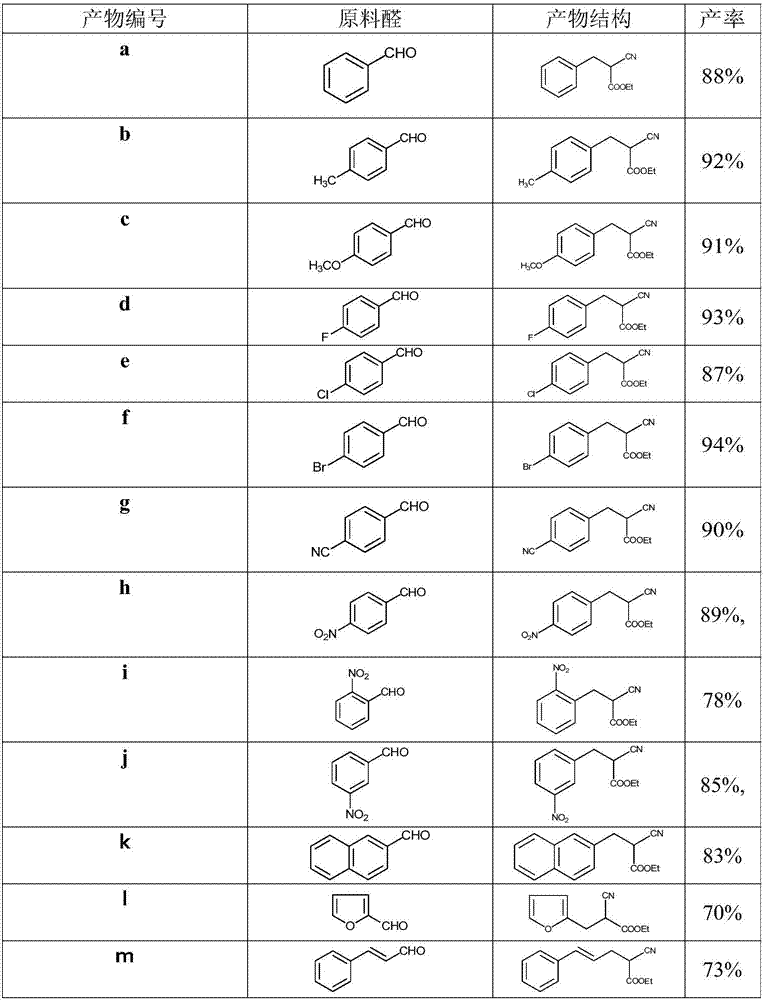
Solvent-free preparation method for substituted cyanoacetate compound
A cyanoacetate, solvent-free technology, applied in the field of preparation of substituted cyanoacetate, can solve the problems of low yield, organic solvent pollution, etc., and achieve the effect of green preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 synthetic method of the present invention
[0023]
[0024] Add 4-bromobenzaldehyde (1) (0.2mmol, 37.0mg), ethyl cyanoacetate (2) (0.24mmol, 27.1mg), dihydropyridinate (0.24mol, 60.7mg) into a 10mL reaction tube, Raise the temperature to 100°C, stir and react for 2 hours, cool down, and then silica gel column chromatography (petroleum ether: ethyl acetate = 10:1 elution), the final product (3) is a colorless liquid with a yield of 68%, NMR The test results are as follows:
[0025] 1 HNMR (400MHz, CDCl 3 ):1.33(t,J=7.16Hz,3H),3.18-3.29(m,2H),3.74(dd,J=5.92,8.14Hz,1H),4.29(q,J=7.14Hz,2H),7.19 (d,J=8.32Hz,2H).
Embodiment 2
[0026] Embodiment 2 synthetic method of the present invention
[0027] 4-Bromobenzaldehyde (1) (0.2mmol, 37.0mg), ethyl cyanoacetate (2) (0.24mmol, 27.1mg), dihydropyridinate (0.24mol, 60.7) and sodium bicarbonate (0.02mmol , 1.68mg) into a 10mL reaction test tube, heated to 100 ° C, stirred for 1 hour, and then silica gel column chromatography (petroleum ether: ethyl acetate = 10:1 elution), finally a colorless liquid product (3 ), yield 72%.
Embodiment 3
[0028] The screening of base in the synthesis method of the present invention in embodiment 3
[0029] According to the method in Example 2, the present invention has screened the kind of alkaline reagent in the synthesis method, and the results are shown in Table 1.
[0030] The screening of alkaline reagent in the synthetic method of the present invention in table 1
[0031] alkaline reagent
[0032] The results show that when the synthesis method of the present invention adds alkali to the reaction raw materials, the yield is obviously better than that of the synthesis method without alkali in Example 1. Among them, DEAE and DABCO have better effects.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com