Pyropheophorbide-a methyl ether compounds, and preparation method and application thereof
A technology of pyropheophorbide and its compound, which is applied in the field of pyropheophorbide a methyl ether compound and its preparation, can solve the problems of high selling price, high production cost, difficulty in preparing taraporfin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
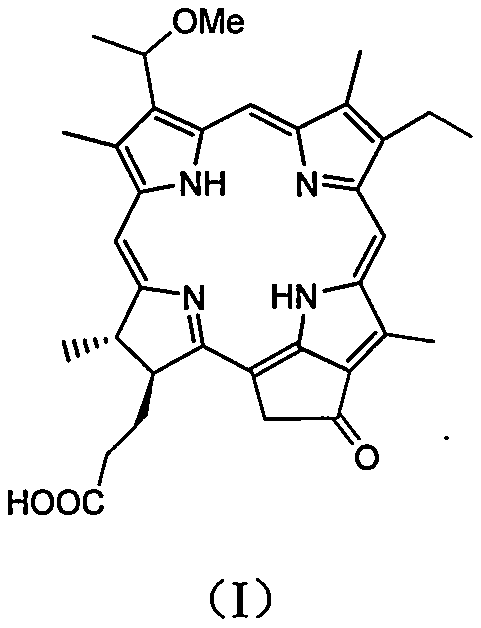
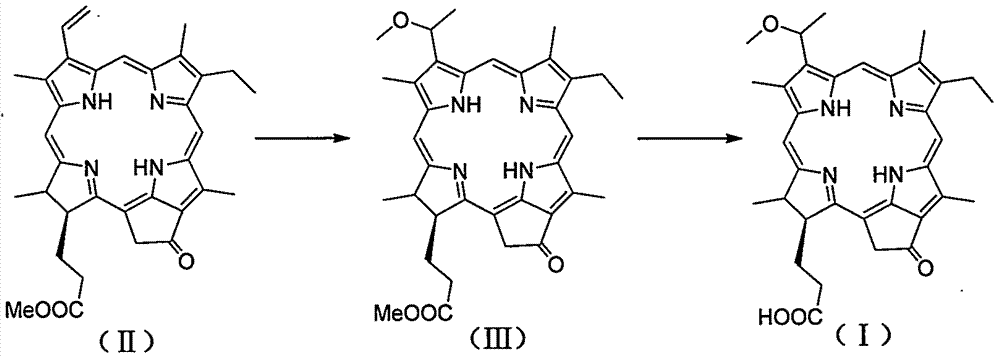
[0021] 1, the preparation method of 3-(1-methoxyethyl)-3-desvinyl pyropheophorbide a
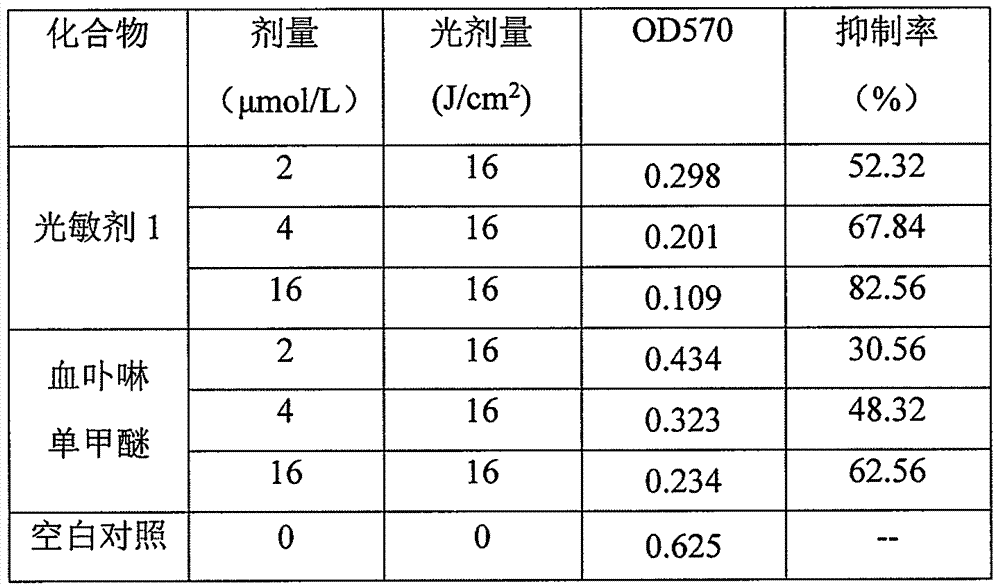
[0022] Dissolve 230mg (0.4mmol) of pyropheophorbide-a methyl ester in 20ml of methanol, add 5mL of acetic acid solution containing 30-35% hydrogen bromide, and react at 40°C for 3 hours. Concentrate under reduced pressure, add 10 mL of methanol to the residue, and continue stirring at room temperature for 8 hours. Add 20 mL of 2M LiOH solution, and stir at room temperature for 5 hours. Add AcOH to the reaction solution to adjust the pH to 5-6; add 60mL CH 2 Cl 2 The reaction solution was extracted; the organic phase was washed with saturated brine. The organic phase was collected, dried over anhydrous magnesium sulfate, and filtered; the filtrate was concentrated; the resulting residue was subjected to column chromatography (dichloromethane / methanol=30 / 1) to obtain 212 mg of a dark brown product with a yield of 79.4%. 1 H NMR (400MHz, CDCl 3 ): δ9.70, 9.50, 8.56 (each s, 1H, meso-H), 5....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com