Polysubstituted indole bithiophene and derivative and synthesis method thereof
A synthetic method and multi-substitution technology, applied in the field of organic compound synthesis, can solve problems such as complex synthesis steps, achieve the effects of saving raw materials, cheap and easy-to-obtain reaction raw materials, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-46
[0089] Step 1: Add indole compounds (see Table 1 for specific substances), alkene (alkynes, ketones) compounds (see Table 1 for specific substances) and sulfur powder into the reaction vessel, and add Bronsted acid (see Table 1 for specific substances) ) solution can also be added to the reaction vessel in the container respectively to Bronsted acid (see Table 1 for specific substances) and organic solvent (see Table 1 for specific substances);
[0090] Step 2: The reaction vessel is evenly heated (such as heating in an oil bath) to the temperature described in Table 1, and the indole compounds, alkene (yne, ketone) compounds and sulfur powder are reacted in a solvent, and continue in Table 1. the time mentioned;
[0091] Step 3: Purification step
[0092]Table 1: Indole compound, alkene (alkyne, ketone) compound, Bronsted acid, indole compound, alkene (alkyne, ketone) compound, sulfur powder and Bronsted acid in embodiment 1-46 Molar ratio, reaction temperature and reaction...
Embodiment 1
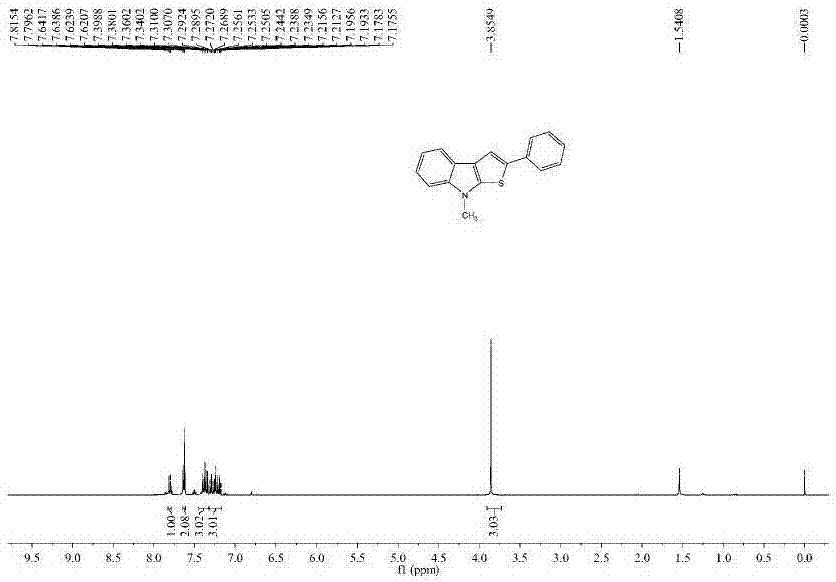
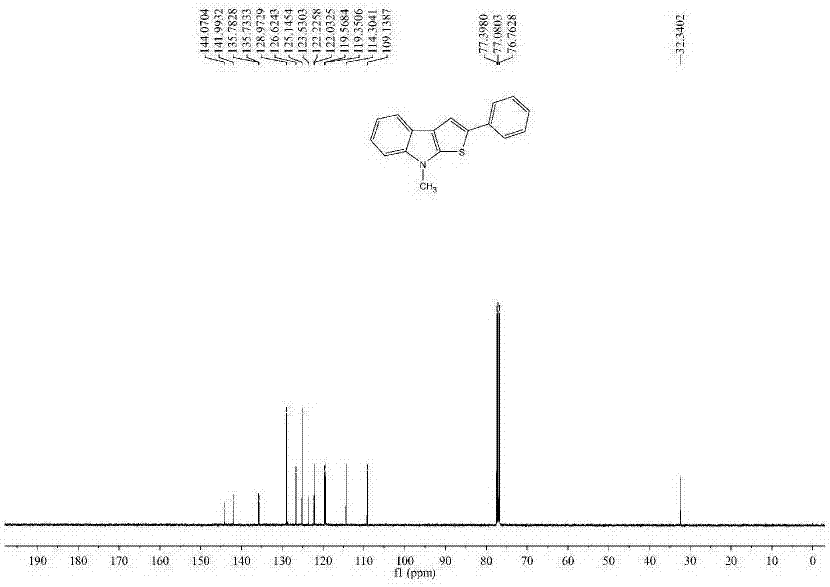
[0100] The nuclear magnetic data of embodiment 1 product is as follows:
[0101] 1H NMR (100MHz, CDCl3, ppm): δ7.80 (d, J = 7.8Hz, 1H), 7.64–7.62 (m, 3H), 7.37 (dd, J = 15.7, 7.7Hz, 3H), 7.31–7.27 (m,1H),7.25–7.23(m,1H),7.21–7.17(m,1H),3.85(s,3H);13C NMR(100MHz,ppm):δ144.1,142.0,135.78,135.73,129.0,126.6 ,125.1,123.5,122.2,122.0,119.6,119.4,114.3,109.1,32.4.
Embodiment 2
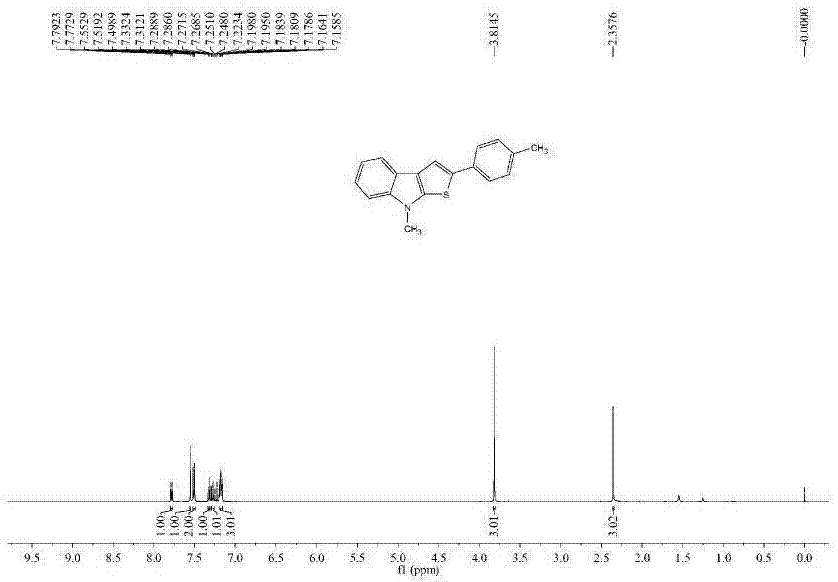
[0102] The nuclear magnetic data of embodiment 2 product is as follows:
[0103] 1H NMR (100MHz, CDCl3, ppm): δ = 7.78 (d, J = 7.8, 1H), 7.55 (s, 1H), 7.51 (d, J = 8.2, 2H), 7.33–7.31 (m, 1H), 7.29–7.25(m,1H),7.20–7.16(m,3H),3.81(s,3H),2.36(s,3H);13C NMR(100MHz,CDCl3,ppm):δ143.8,142.0,136.5,136.0, 133.0, 129.6, 125.1, 123.5, 122.2, 121.9, 119.5, 119.3, 113.7, 109.1, 32.3, 21.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com