Linaclotide purifying method
A technology of linaclotide and purification method, which is applied in the field of linaclotide purification, can solve problems affecting product purity and content, impurity is difficult to remove, purification effect is not ideal, etc., and achieves simple operation, low impurity content, good The effect of economic and practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Processing of crude linaclotide
[0027] Weigh 0.5g of crude linaclotide, add 10mL of pure water, mix evenly and place in an ultrasonic cleaner for about 10min to promote the dissolution of the sample, absorb the supernatant of the mixed system, and use a 0.22μm PVDF membrane to remove the insoluble Impurities.
[0028] 2. Purification of Linaclotide Samples
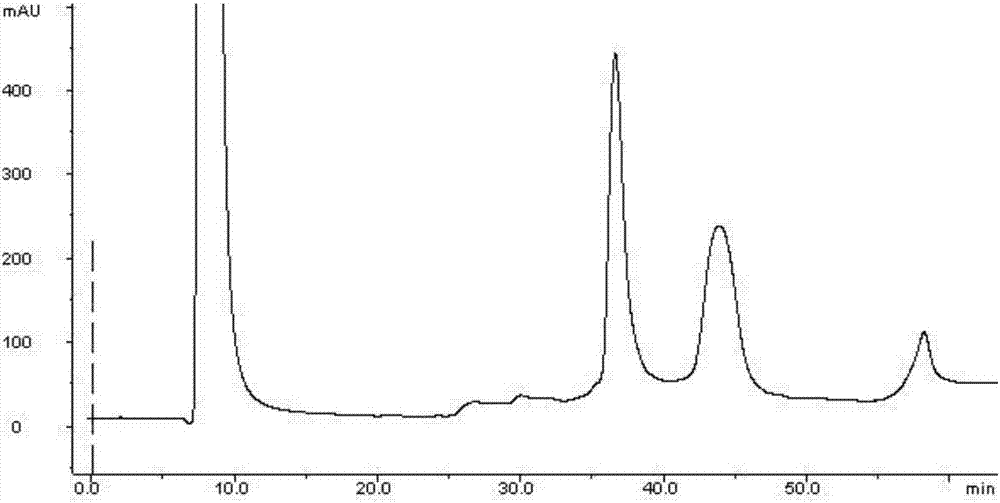
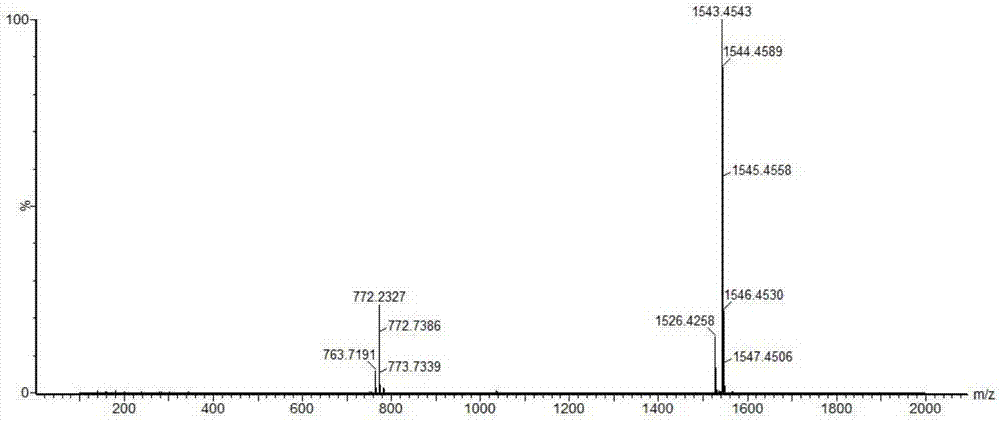
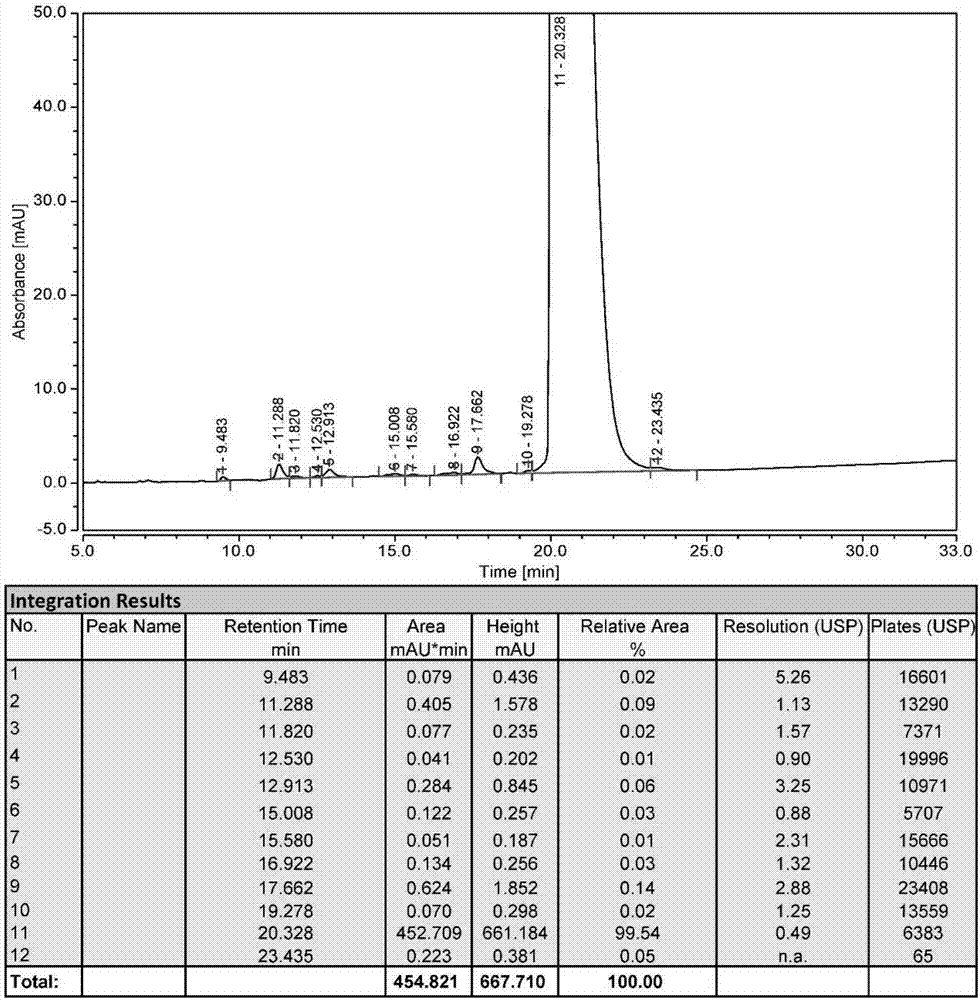
[0029] Use the anion exchange column SOURCE 15Q as the separation medium, after loading the crude product solution, use the 100mmol / L Tris-HCl buffer solution containing pH8.5 as the mobile phase A, and use the pH8.5 Tris-HCl buffer solution containing 1 mol / L NaCl The mobile phase B was used for gradient elution, and the eluate was collected; the system was flushed with mobile phase A first, and the sample was loaded after equilibration. The flow rate was 0.5mL / min, and the ratio of mobile phase A and mobile phase B was adjusted to 50 within 30 minutes. % for elution. After the sample peaks, adjust the prop...
Embodiment 2
[0033] 1. Processing of crude linaclotide
[0034] Weigh 0.5g of crude linaclotide, add 10mL of pure water, mix evenly and place in an ultrasonic cleaner for about 10min to promote the dissolution of the sample, absorb the supernatant of the mixed system, and use a 0.22μm PVDF membrane to remove the insoluble Impurities.
[0035] 2. Purification of Linaclotide Samples
[0036] Anion exchange column Q-SepharoseFast Flow was used as the separation medium. After loading the crude product solution, 80mmol / L Tris-HCl buffer solution containing pH8.2 was used as mobile phase A, and pH8.2 Tris-HCl buffer solution containing 0.8mol / L NaCl was used as mobile phase A. HCl buffer is mobile phase B for gradient elution, collect the eluate; first use mobile phase A to flush the system, and load the sample after equilibration, the flow rate is 0.5mL / min, adjust the ratio of mobile phase A and mobile phase B within 30min to 50% for elution. After the sample peaks, adjust the proportion of...
Embodiment 3
[0040] 1. Processing of crude linaclotide
[0041] Weigh 0.5g of crude linaclotide, add 10mL of pure water, mix evenly and place in an ultrasonic cleaner for about 10min to promote the dissolution of the sample, absorb the supernatant of the mixed system, and use a 0.22μm PVDF membrane to remove the insoluble Impurities.
[0042] 2. Purification of Linaclotide Samples
[0043]Use the anion exchange column SOURCE 15Q as the separation medium, after loading the crude product solution, use the 120mmol / L Tris-HCl buffer solution containing pH8.8 as the mobile phase A, and use the pH8.8 Tris-HCl buffer solution containing 1.2mol / L NaCl The mobile phase B was used for gradient elution, and the eluate was collected; the system was flushed with mobile phase A first, and the sample was loaded after equilibration. The flow rate was 0.5mL / min, and the ratio of mobile phase A and mobile phase B was adjusted to 50 within 30 minutes. % for elution. After the sample peaks, adjust the prop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com