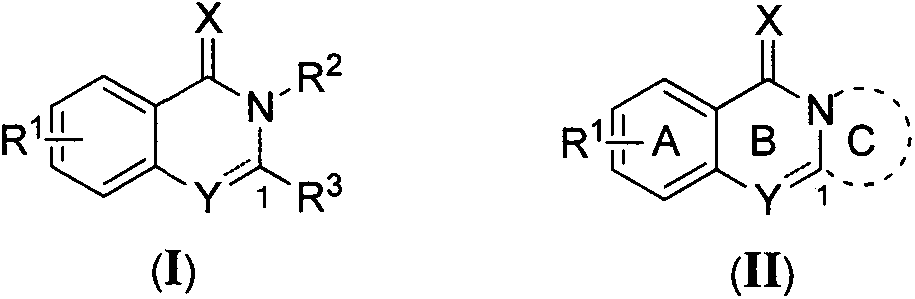
Isoquinolone derivative as well as preparation method and application thereof
A technology of isoquinolinone and its derivatives, which is applied in the field of preparation of isoquinolinone derivatives and their pharmacologically acceptable salts, esters, prodrugs, and can solve the problem of harsh reaction conditions, high cost and low yield And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Synthesis of some compounds of the present invention, including Ia-Il and IIa-IIc, taking compound Ia as an example.
[0037] N, the preparation of N-dibenzyl-2-hydroxybenzamide:
[0038]
[0039] Dissolve 1 g (7.25 mmol) of salicylic acid in 30 mL of anhydrous dichloromethane, add 1.05 mL (14.5 mmol) of thionyl chloride, 1 drop of N,N-dimethylformamide, and heat up to reflux for 2 hours. Cool to room temperature, spin dry the solvent, dissolve in anhydrous dichloromethane, and add dropwise to anhydrous dichloromethane solution containing 3.10mL (21.75mmol) of triethylamine and 1.48g (7.25mmol) of dibenzylamine. After the dropwise addition was completed, the mixture was stirred at room temperature for 5 min, and spin-dried to obtain a crude product, which was purified by column chromatography to obtain 1.95 g of white solid N,N-dibenzyl-2-hydroxybenzamide, with a yield of 85%. 1 H NMR (300MHz, CDCl 3 )δ4.72(s, 4H), 6.77(td, J=7.6, 1.2Hz, 1H), 6.99-7.10(m,...
Embodiment 2
[0085] Example 2 Antitumor Activity Experiment of Compounds Ia-Il, IIa-IIc.
[0086] Experimental Materials:
[0087] Cell line: human cervical cancer cell HeLa and human breast cancer cell MCF-7; sample: compound Ia-Il, IIa-IIc added appropriate amount of DMSO to make the concentration of 10 -2 M solution, diluted with culture medium to the corresponding concentration before use.
[0088] experimental method:
[0089] Cell digestion and counting, 2.5×10 per well 3 Spot cells in a 96-well plate, with a total volume of 100 μL per well, culture for 24 hours, dilute the sample to be tested to an appropriate concentration with medium, 100 μL per well, set up 3 duplicate wells, add 20 μL of 5 mg / mL MTT solution after 72 hours of culture After incubation for 4 hours, suck out all the liquid in the well, add 100 μL DMSO, and measure the absorbance with a full-wavelength microplate reader. Calculate the inhibition rate according to the absorbance value, and the calculation formula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com