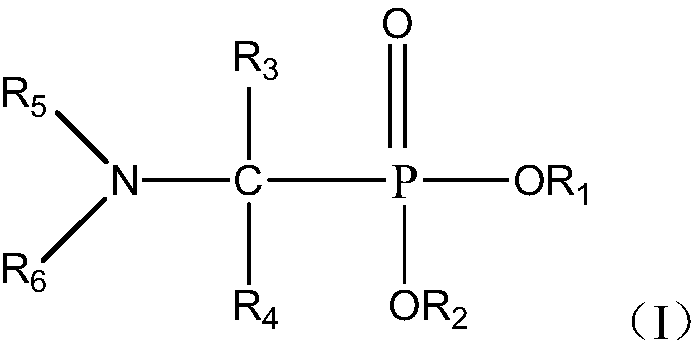
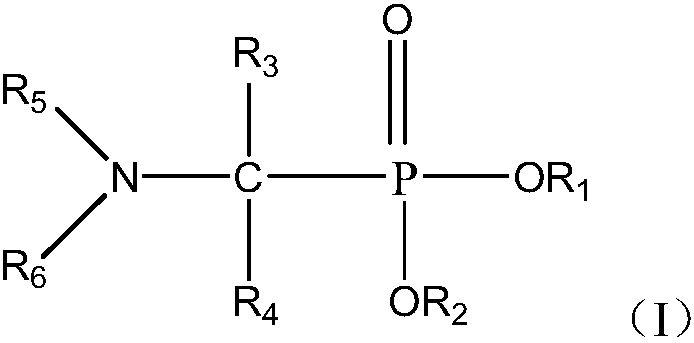
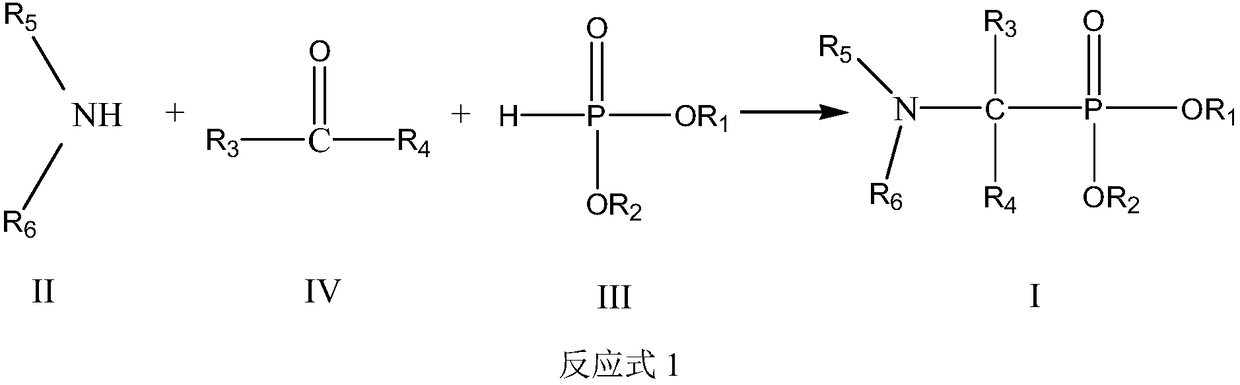
Use and method of neutral phosphine extractant for extracting and separating cerium (iv) or thorium (iv)
An extractant and neutral phosphine technology, which is applied in the field of extraction and separation of tetravalent cerium (quaternary cerium) or tetravalent thorium), can solve the problems of high extraction and separation costs, low economic benefits, and difficult synthesis, so as to reduce extraction and separation costs and cost The effect of low cost and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0104] In order to further illustrate the solutions of the present invention, specific examples of the present invention are provided to help those skilled in the art understand and implement the present invention, but the present invention is not limited to these examples.
[0105] Preparation Example
[0106] Reagents and sources
[0107] Bis(2-ethylhexyl) phosphite, diethyl phosphite, aviation kerosene and TBP were purchased from Shanghai Laiyashi Chemical Co., Ltd.
[0108] Metaldehyde, propionaldehyde, acetone, di-n-hexylamine, diisobutylamine, n-butylamine, diisooctylamine, dodecylamine, isooctylamine, dimethylamine, toluene, xylene, p-methyl Benzenesulfonic acid and heptane were purchased from Aladdin Reagent Co., Ltd.
[0109] Cyanex 923 and 2-methylheptanol were purchased from Shanghai Cyanex Chemical Co., Ltd.
[0110] Feed liquid, washing liquid and stripping agent are self-made in the laboratory.
[0111] Other reagents (such as acids, etc.) are commercially av...
preparation example 1
[0114] Preparation Example 1: Preparation of 1-(2-ethylhexylamino)-1-methylethylphosphonic acid bis(2-ethylhexyl)ester
[0115]
[0116] In a single-necked flask equipped with a mechanical stirrer and a condensing reflux device, add bis(2-ethylhexyl) phosphite (1mol), acetone (1.1mol), isooctylamine (1.1mol), toluene (800ml) and P-toluenesulfonic acid (0.8g), stirred and refluxed, reacted at 80-90°C for 12h. Toluene and unreacted raw materials were removed by rotary evaporation to obtain the target product.
[0117] 1 H NMR (600MHz, CDCl 3 , ppm): δ0.84-1.54[m, (CH 3) 8 ,(CH 2 ) 12 ,(CH) 3 ],2.59[d,(CH 2 )],3.98[m,(CH 2 ) 2 ].
preparation example 2
[0118] Preparation Example 2: Preparation of 1-(N,N-diisobutylamino)-1-methylethylphosphonic acid bis(2-ethylhexyl)ester
[0119]
[0120] Except that diisobutylamine was used instead of isooctylamine, the target product was prepared by the same process as in Preparation Example 1.
[0121] 1 H NMR (400MHz, CDCl 3 ,ppm): δ0.89-1.71[m,(CH 3 ) 10 ,(CH 2 ) 8 ,(CH) 2 ],2.06[m,(CH) 2 ],2.32[m,(CH 2 ) 2 ],4.12[m,(CH 2 ) 2 ].
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bronsted acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com