Water soluble fatty acid imidazoline and preparation method and application thereof
A technology of fatty acid imidazoline and fatty acid imidazoline, which is applied in the field of fatty acid derivatives and their synthesis technology, can solve the problems of poor extreme pressure wear resistance and corrosion resistance, low water viscosity, and difficulty in forming a fluid lubricating film, etc., to achieve Save energy and resources, simple preparation process, easy to operate and prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Add 26.23g of 6-aminocaproic acid, 12.38g of diethylenetriamine, and 50ml of xylene into a 250ml four-necked bottle as a solvent and a water-carrying agent. React at 140-160°C for 4 hours to remove the water generated in the reaction, react at 190-210°C for 2 hours to remove the water generated in the reaction, and then distill under reduced pressure for 1 hour to remove unreacted raw materials, and then obtain brown viscous liquid diethylene Triamine imidazoline.
Embodiment 2
[0051] Example 2: 26.23g of 6-aminocaproic acid was added to a 250ml four-neck flask, 25.07g of triethylenetetramine (purity of 70% by mass) was added, and 50ml of xylene was added as a solvent and a water-carrying agent. React at 140-160°C for 5 hours to remove the water generated in the reaction, react at 190-210°C for 3 hours to remove the water generated in the reaction, and then distill under reduced pressure for 1 hour to remove unreacted raw materials, and then obtain brown viscous liquid triethylene Tetraamine imidazoline.
Embodiment 3
[0052] Example 3: Add 26.23g of 6-aminocaproic acid, 22.72g of tetraethylenepentamine, and 50ml of xylene into a 250ml four-necked bottle as a solvent and a water-carrying agent. React at 140-160°C for 6 hours to remove the water generated in the reaction, react at 190-210°C for 2 hours to remove the water generated in the reaction, and then distill under reduced pressure for 1 hour to remove unreacted raw materials, and then obtain brown viscous liquid tetraethylene Pentaamine imidazoline.
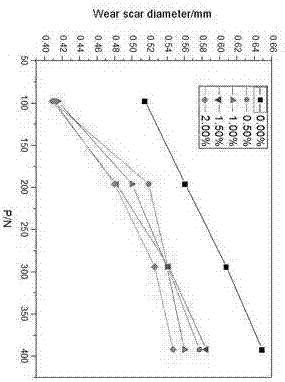
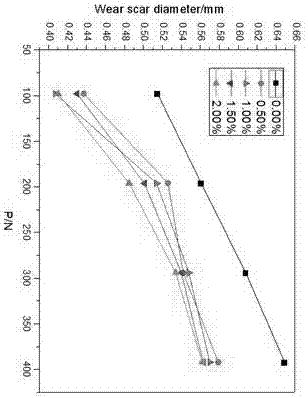
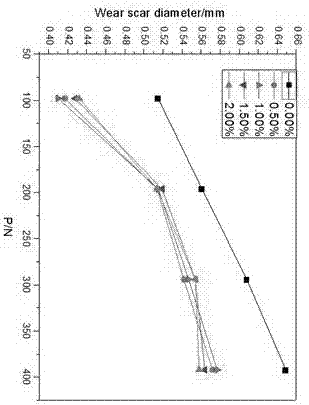
[0053] All target compounds were determined by Spectrum One infrared spectrometer to have imidazole rings in the synthesized compounds. The C, H, and N element analysis results of the additives are shown in Table 1. From the elemental analysis results in Table 1, it can be seen that the measured values of C, H, and N elements of all target compounds are basically consistent with the theoretical values determined according to the molecular formula, and the absolute error is within the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com