N-acyl homoserine lactonase and medicine thereof
A technology of acyl homoserine lactone and acyl homoserine, which is applied in the field of N-acyl homoserine lactonase and its medicine, and can solve the problems of life-threatening, difficult to cure, severe infection and other problems of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
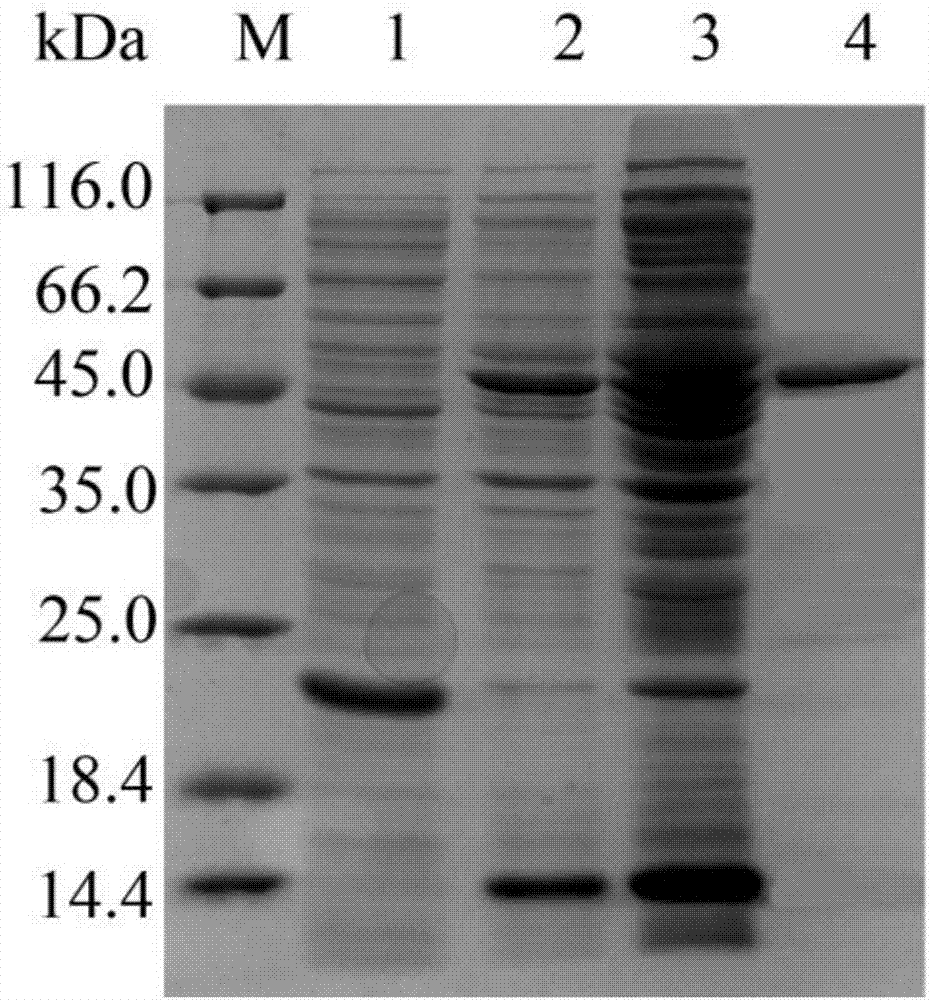
[0030] This example is used to illustrate the purification process of AiiK protein.
[0031] Using the K. huagui DNA with the preservation number of ACCC 06121 as the amplification template, use the primer pair such as SEQ ID NO.3 and SEQ ID NO.4 to amplify to obtain the amplified product; use EcoRI and HindⅢ endonuclease The amplified product was digested and cloned into the expression vector pET32a to obtain the pET32a-aiiK prokaryotic expression plasmid; the above pET32a-aiiK prokaryotic expression plasmid was introduced into E. coli BL21 cells to obtain the E.coli BL21 / pET32a-aiiK prokaryotic expression strain .
[0032] The E.coli BL21 / pET32a-aiiK prokaryotic expression strain was shaken in LB medium (containing 100 μg / mL ampicillin) at 37°C and 180r / min until OD 600 ≈0.6, add IPTG with a final concentration of 0.5mM, and continue to cultivate at 25°C and 120r / min for 12h to obtain culture products. The control group was the culture product obtained by culturing the E. ...
Embodiment 2
[0036] This example measures the enzyme activity of AiiK at 25°C.
[0037] In this embodiment, N-decanoyl homoserine lactone (C 10 -HSL) was used as the substrate to measure the enzyme activity. According to the reaction system (N-HSL 2.5 μL, the final concentration of AiiK is 4 μg / mL, add 10 mM PBS to make the total volume 500 μL), add each component into a 2 mL centrifuge tube and mix well, react at 25 °C for 10 min, and replace AiiK with 10 mM PBS in the blank control group Protein, after the reaction, immediately add a volume of 55.5 μL of 20% SDS (final concentration is 2%) to the centrifuge tube to terminate the reaction; add ethyl acetate to the centrifuge tube and vortex extract the reaction product, the extracted reaction The product was concentrated and dried under flowing nitrogen, then redissolved in methanol, passed through a 0.22 μm filter membrane, and determined by HPLC. 10 -HSL content and calculate enzyme activity. The unit of enzyme activity (U) is to deg...
Embodiment 3
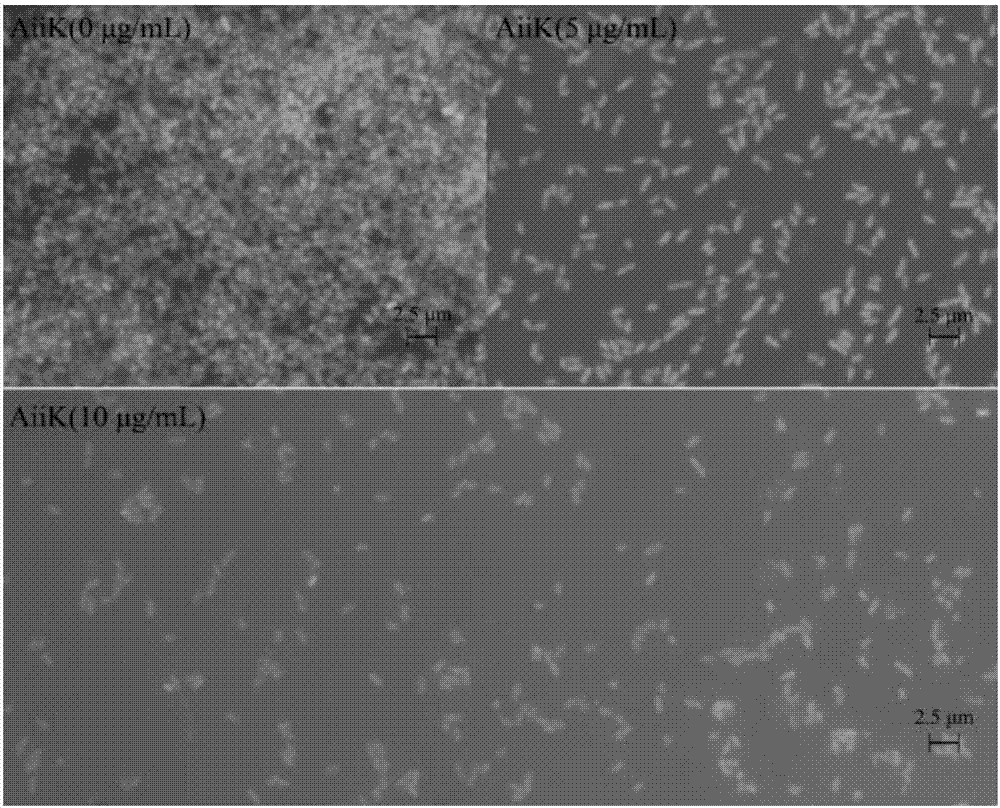
[0040] In this example, the effects of temperature and other enzymes on the activity of AiiK enzymes were determined.
[0041] According to the method described in Example 2, the enzyme activity of AiiK after incubation at 37°C and 45°C for 2h, 4h, 6h, 12h, 24h, and 36h (calculated as 100% of the enzyme activity at 0h) was measured respectively. For specific results, see Table 1.
[0042] According to the method described in Example 2, the effects of chymotrypsin, trypsin or proteinase K on the AiiK enzyme activity when the AiiK protein was treated for 30 min and 60 min respectively were determined. In the control group, 10 mM PBS was used instead of chymotrypsin and the enzyme activity was counted as 100%. The specific results are shown in Table 2.
[0043] Table 1
[0044]
[0045] Table 2
[0046]
[0047] In Table 1, when the AiiK protein was incubated at 37°C and 45°C for 2 hours, the enzyme activity remained above 86%, at 6 hours, the enzyme activ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com