Raphanin-containing gastric retention composition and preparation method thereof
A technology for composition and gastric retention, which is applied in the direction of drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of inability to guarantee the stability of the active ingredient radishin and poor drug efficacy, and achieve prolonged direct action Time and drug concentration, improve drug efficacy, ensure stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] In another aspect, on the basis of the above-mentioned embodiments of the present invention, the embodiments of the present invention provide a preparation method of the above-mentioned gastric retention composition. Described preparation method comprises the steps:
[0049] Step S01: according to the components contained in the gastric retention composition described above;
[0050] Step S02: performing mixing treatment on the weighed rapaidulin, hydrophilic gel skeleton, bleaching aid and / or adhesive agent, expansion agent and regulator, and part of the glidant to obtain a mixture;
[0051] Step S03: monitor the moisture content of the mixture;
[0052] Step S04: Sieve and granulate the mixture that meets the moisture monitoring requirements, then mix it with the weighed remaining glidant, and then carry out formulation shaping treatment.
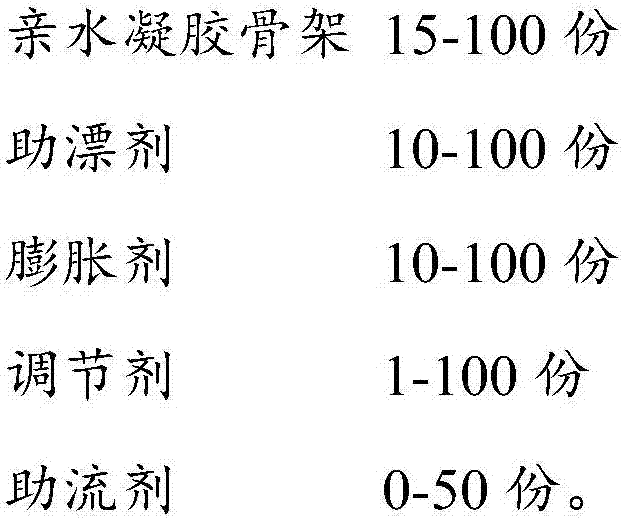
[0053] Wherein, the components contained in the gastric retention composition and the content of each component in the above st...
Embodiment 1
[0062] Embodiment 1 (dry granulation)
[0063] This embodiment provides a gastric retention composition and a preparation method thereof. The gastric retention composition comprises the following components by weight:
[0064] Ratoxine, HPMC, NaHCO 3 , Cetyl Alcohol, Sodium Starch Carboxymethyl, Citric Acid, Micronized Silica Gel. Its weight ratio is 25:100:25:60:30:25:5.
[0065] The preparation method of the gastric retention composition of the present embodiment is as follows:
[0066] S11: According to the ratio of each component of the gastric retention composition in this embodiment, weigh radishin and the above-mentioned auxiliary materials;
[0067] S12: Fully mix the weighed rapaidulin, regulator, and micropowder silica gel, then add hydrophilic gel skeleton, bleaching aid, expansion agent, etc., fully mix, and pass through a 60-mesh sieve to obtain a mixed material;
[0068] S13: Dry granulating the mixed material to prepare a dosage form green body;
[0069] S...
Embodiment 2
[0071] Embodiment 2 (direct compression tablet)
[0072] This embodiment provides a gastric retention composition and a preparation method thereof. The gastric retention composition comprises the following components in parts by weight: radish, HPMC, NaHCO 3 , Cetyl Alcohol, Sodium Starch Carboxymethyl, Citric Acid, Micronized Silica Gel. Its weight ratio is 25:100:25:60:30:25:5.
[0073] The preparation method of the gastric retention composition of the present embodiment is as follows:
[0074] S11: According to the ratio of each component of the gastric retention composition in this embodiment, weigh radishin and the above-mentioned auxiliary materials;
[0075] S12: Fully mix the weighed rapaidulin, regulator, and micropowder silica gel, then add hydrophilic gel skeleton, bleaching aid, expansion agent and other auxiliary materials, fully mix, and pass through a 60-mesh sieve to obtain a mixed material ;
[0076] S13: Carry out moisture monitoring, so that the moistur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com