Method for preparing (S)-tetrahydro-1-naphthoic acid through high-efficiency resolution
A technology of tetrahydronaphthoic acid and naphthoic acid, applied in carboxylate preparation, organic chemical methods, chemical instruments and methods, etc., can solve problems such as environmental pollution, waste of resources, increase production costs, etc., and achieve improved resolution yield , reduce pollution and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
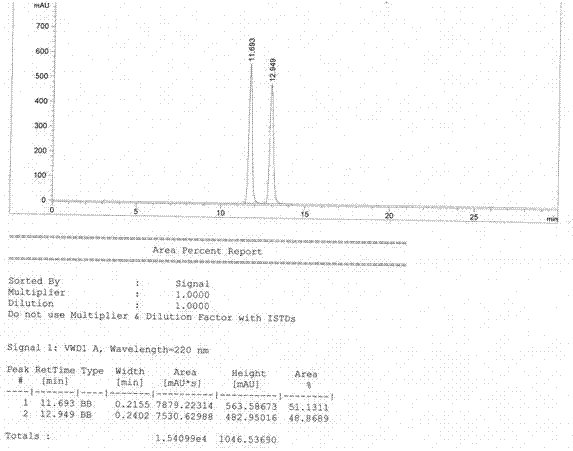
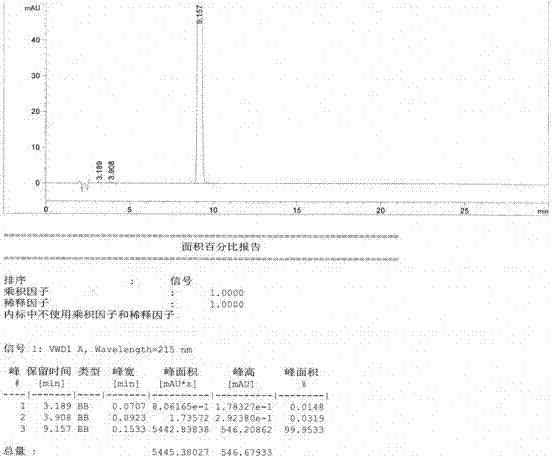
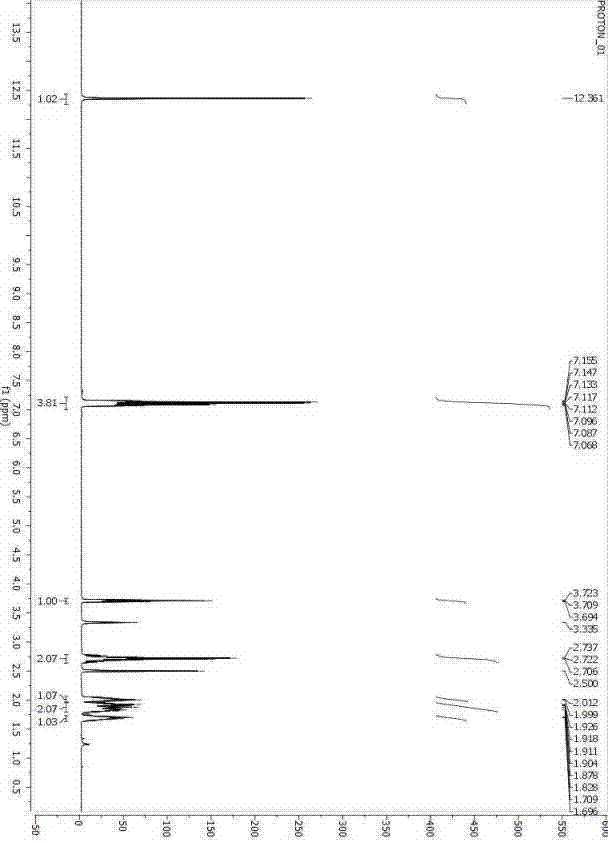
Image
Examples
Embodiment 1
[0025] Embodiment 1: ( S )-1,2,3,4-tetrahydro-1-naphthoic acid preparation
[0026] (1) Add 4500ml of ethanol and 750ml of water into a 10L three-necked bottle, and add 250g of 1,2,3,4-tetrahydronaphthoic acid and 462g of quinine in batches. Warming to reflux and stirring for 2h. After that, the temperature was lowered to about 0°C and stirred for 12 hours. Filter and collect the filtrate for later use. The filter cake was air-dried in an oven at 60° C. to dryness to obtain 325 g of a white solid, namely compound III.
[0027] (2) Add 1250ml of ethyl acetate to the white solid compound III obtained above, and then add 625ml of 2mol / L hydrochloric acid aqueous solution, stir for 30min, and let stand to separate layers. The upper organic phase was dried by adding 100 g of anhydrous sodium sulfate, then placed in a water bath at 50°C and concentrated to dryness to obtain 110 g of a white powdery solid, namely compound I.
[0028] (3) Put the filtrate collected in the above r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com