Perylene-based small molecular fluorescent probe and preparation method and application thereof
A fluorescent probe and small molecule technology, applied in the field of perylene-based small molecule fluorescent probe and its preparation, can solve the problems of poor photobleaching resistance, high detection cost, high biological toxicity, etc., and achieve selective biological low toxicity, selective Good performance and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The preparation process of the small molecule fluorescent probe of this embodiment mainly includes the following steps:
[0058] 1. Place 0.5g (2mmol) of perylene and 40mL of 1,4-dioxane in a double-necked flask in sequence, and stir at 80°C until the perylene is completely dissolved into a clear yellow solution; then slowly inject 1mL of concentrated Nitric acid, concentrated nitric acid concentration is 65%~68% concentrated nitric acid (14.4~15.2mol / L), after stirring at 60 ℃ for 30min, obtain the reaction solution comprising compound 1 and compound 2; Pour into a large amount of ice water, filter under reduced pressure and vacuum dry for 12 hours to obtain a bright red crude product; finally, use petroleum ether (PE)-dichloromethane (DCM) with a volume ratio of 1.5:1 as the eluent by chromatography The dark red needle-like solid compound 1 (yield 20%) and brick red solid compound 2 (yield 63%) were obtained.
[0059] Its chemical reaction formula is as follows:
[...
Embodiment 2
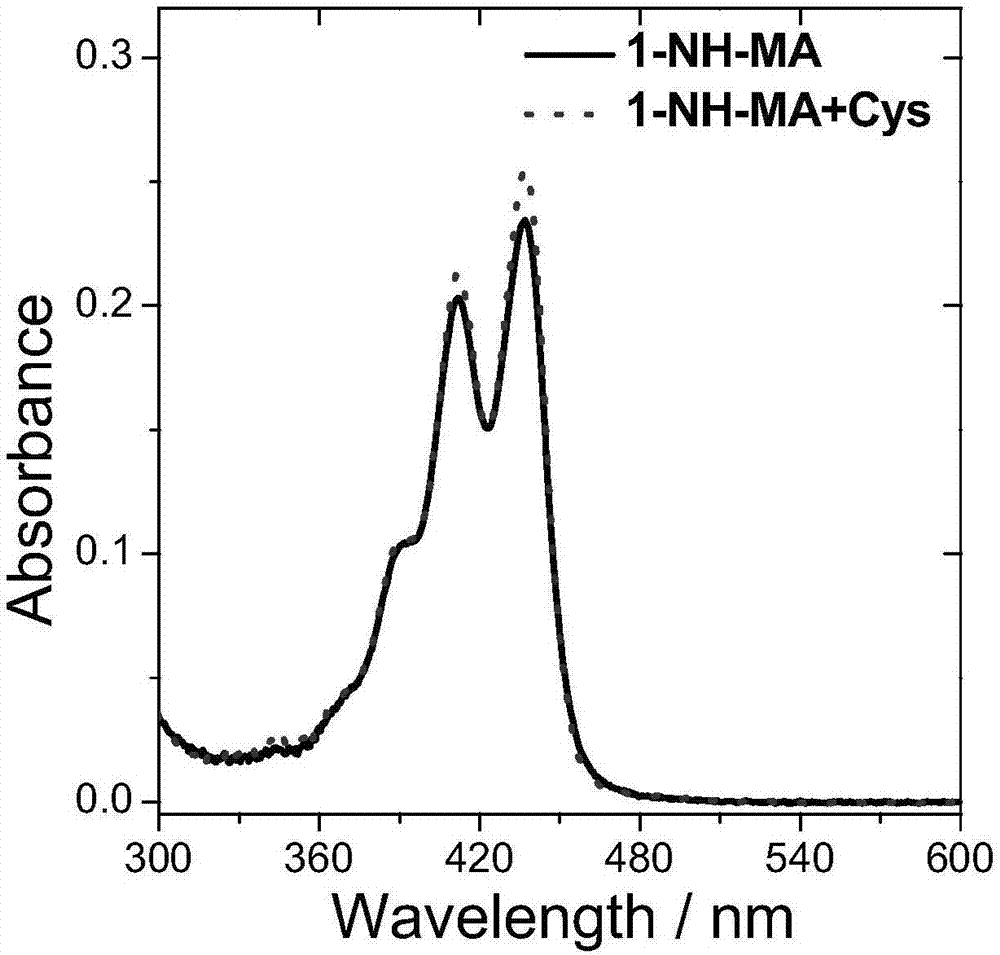
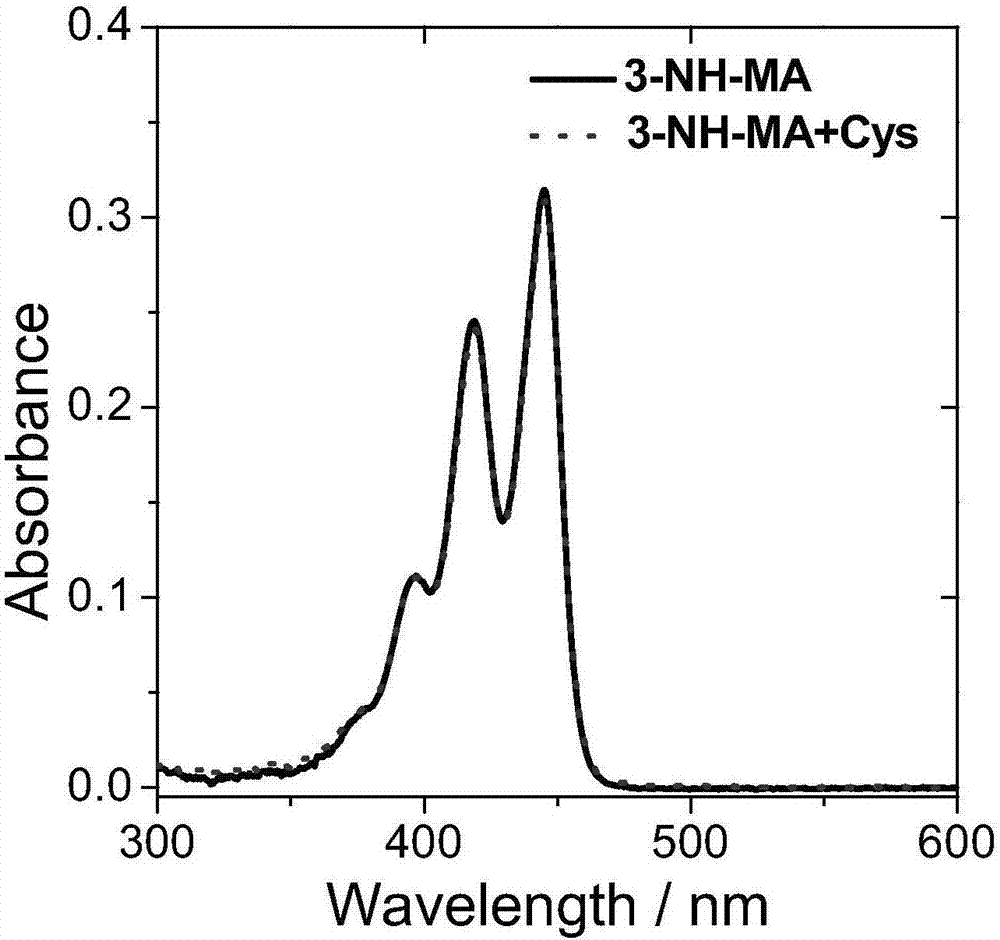
[0085] Compound 5 and compound 6 were named as fluorescent probes 1-NH-MA and 3-NH-MA respectively, and then both were prepared with DMSO at a concentration of 10 -5 mol / L probe solution; then, add cysteine solution (Cys) with the same concentration as the probe to the two probe solutions respectively; finally, use a UV-visible spectrophotometer to detect its absorbance. The result is as figure 1 and figure 2 As shown, the absorptions of probes 1-NH-MA and 3-NH-MA are both located between 350nm and 480nm, and the absorption wavelength and absorption intensity of the probes have almost no change before and after adding cysteine.
Embodiment 3
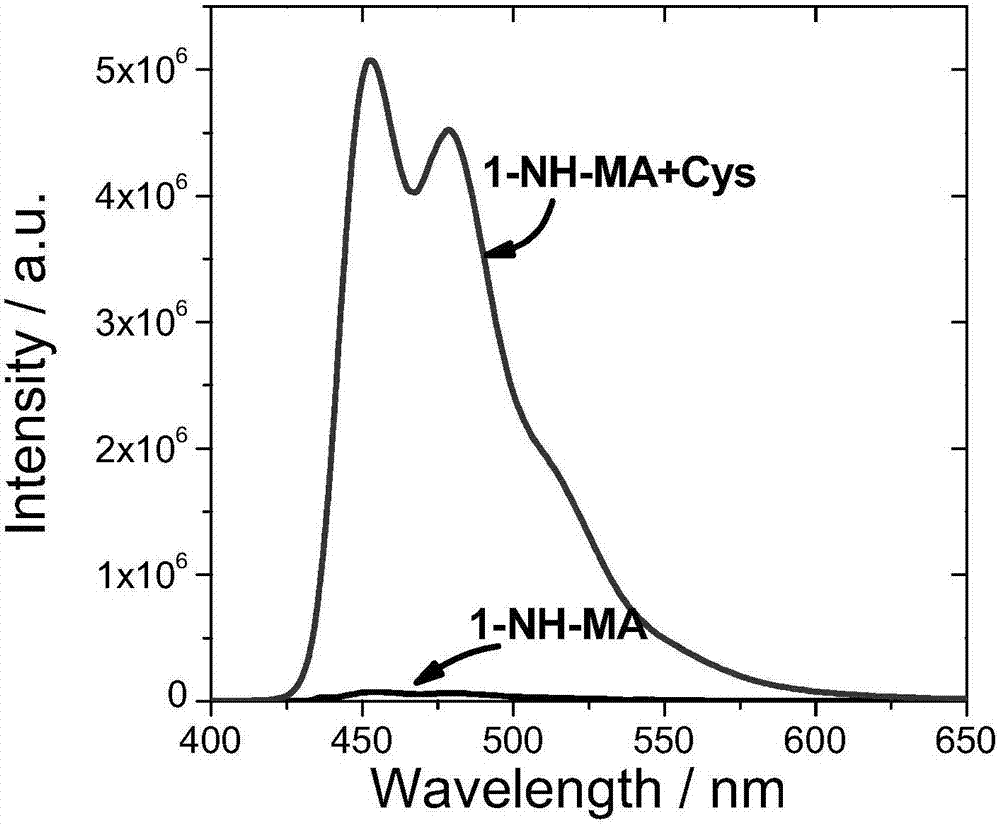
[0087] Compound 5 and compound 6 were named as fluorescent probes 1-NH-MA and 3-NH-MA respectively, and then both were prepared with DMSO at a concentration of 10 -5 mol / L probe solution; then, add cysteine solution (Cys) with different concentrations respectively to two kinds of probe solutions, test the impact of Cys on the fluorescence intensity of the probe, it is found through testing that when the Cys concentration is 1×10 -5 mol / L, the probe has the strongest luminescence intensity. Such as image 3 and Figure 4 As shown, the probes 1-NH-MA and 3-NH-MA themselves do not emit light or emit light relatively weakly, adding Cys(10 -5 mol / L) immediately after the obvious fluorescence enhancement, the fluorescence intensity of 1-NH-MA and 3-NH-MA was enhanced by 71 times and 196 times after Cys was added, indicating that the two fluorescent probes provided in this example have relatively high High sensitivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com