Synthesis method for tetrahydrospiro compound
A synthetic method and compound technology, applied in the direction of organic chemistry, etc., to achieve the effect of wide substrate range, simple reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
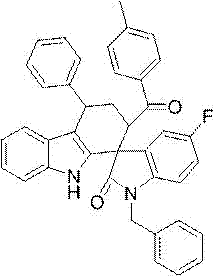
[0028] Example 1: Preparation of ethyl 1'-benzyl-5'-chloro-9-methyl-2'-oxo-4-phenyl-2,3,4,9-tetrahydrospiro[carbazole-1 ,3'-indoline]-2-carboxylate methyl ester as an example, its reaction formula is as follows:
[0029]
[0030] The preparation method is as follows:
[0031] Under the protection of nitrogen, add in a 10 mL Schlenck tube N -Methyl indole (0.5 mmol, 0.066g), acetophenone (1.5 mmol, 0.180g), 2 mL of dry toluene and trifluoromethanesulfonic acid (5 mol%, 0.004g) were stirred at 60°C for 1.5 hours, Then ethyl 2-(1-benzyl-5-chloro-2-oxoindolin-3-ylidene)carboxylate (0.5 mmol, 0.170 g) was added and the mixture was stirred at 80°C for 12 hours. TLC detects the reaction process, when the reaction is over, add 5mL water to the system, extract with ethyl acetate (5mL×3), combine the organic layers, and wash the organic layer with anhydrous Na 2 SO 4 Drying, suction filtration under reduced pressure, rotary evaporation of the filtrate, using ethyl acetate and lig...
Embodiment 2
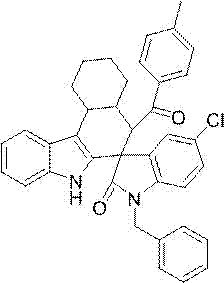
[0034] Example 2: Preparation of 1'-butyl-4-(4-chlorophenyl)-5'-fluoro-9-methyl-2'-oxo-2,3,4,9-tetrahydro with the following structural formula Ethyl spiro[carbazole-1,3'-indoline]-2-carboxylate:
[0035]
[0036] Preparation method: In Example 1, the 2-(1-benzyl-5-chloro-2-oxoindoline-3-ylidene) ethyl carboxylate used was prepared with an equimolar amount of 2-(1 -n-butyl-5-fluoro-2-oxoindoline-3-ylidene) ethyl carboxylate is replaced, acetophenone is replaced with p-chloroacetophenone of equimolar amount, other steps method and example 1 Same, to get ethyl 1'-butyl-4-(4-chlorophenyl)-5'-fluoro-9-methyl-2'-oxo-2,3,4,9-tetrahydrospiro[carba Azole-1,3'-indoline]-2-carboxylic acid, the isolated yield was 79%, 0.220 g.
[0037] The structural characterization data are as follows:
[0038] m.p.239-241℃; 1 H NMR (400 MHz, CDCl 3 ) δ: 7.37 (d, J = 7.6 Hz, 2H, ArH),7.27 (d, J = 7.6 Hz, 2H, ArH), 7.11-7.06 (m, 3H, ArH), 6.90-6.86 (m, 2H,ArH), 6.84-6.82 (m, 1H, ArH), 6.70 (...
Embodiment 3
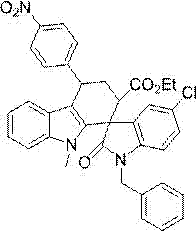
[0039] Example 3: Preparation of 1'-benzyl-5'-chloro-4-(4-methoxyphenyl)-9-methyl-2'-oxo-2,3,4,9- Ethyl tetrahydrospiro[carbazole-1,3'-indoline]-2-carboxylate:
[0040]
[0041] Preparation method: In Example 1, the acetophenone used is replaced with an equimolar amount of p-methoxyacetophenone, and the other steps are the same as in Example 1 to obtain 1'-benzyl-5'-chloro-4- (4-Methoxyphenyl)-9-methyl-2'-oxo-2,3,4,9-tetrahydrospiro[carbazole-1,3'-indoline]-2-carboxy Acetate ethyl ester, its isolated yield was 88%, 0.266 g.
[0042] The structural characterization data are as follows:
[0043] m.p.207-209℃; 1 H NMR (400 MHz, CDCl 3 ) δ: 7.47 (d, J = 7.2 Hz, 2H, ArH),7.37-7.28 (m, 5H, ArH), 7.24 d, J = 8.0 Hz, 1H, ArH), 7.11-7.08 (m, 3H, ArH),6.86 (d, J = 6.8 Hz, 2H, ArH), 6.84-6.80 (m, 2H, ArH), 6.75 (d, J = 7.6 Hz,1H, ArH), 5.23 (d, J = 15.2 Hz, 1H, CH), 4.77 (d, J = 14.8 Hz, 1H, CH), 4.36(dd, J 1 = 11.6 Hz, J 2 = 5.6 Hz, 1H, CH), 3.98-3.83 (m, 2H, CH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com