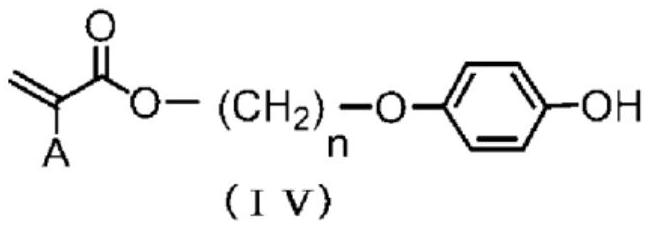Mixture of polymerizable compounds and method for producing the same
A technology of polymeric compounds and manufacturing methods, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of low solubility, narrow temperature range of display liquid crystallinity, insufficient abnormal wavelength dispersion, etc., to achieve Excellent solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0289] (Example 1) Preparation of Mixture 1
[0290] [chemical formula 31]
[0291]
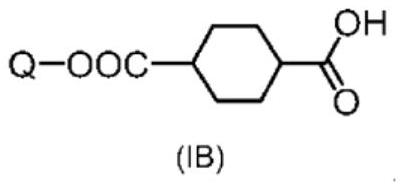
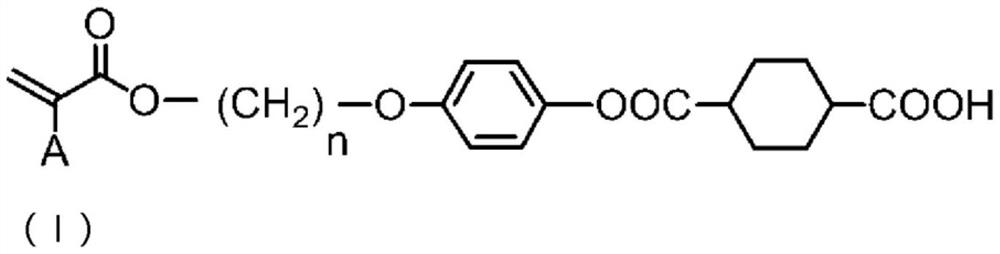
[0292] In a 3-port reactor with a thermometer, 10.0 g (47.83 mmol) of trans-1,4-cyclohexane dicarboxylic acid chloride and cyclopentyl methyl ether ( CPME) 200ml. 12.64 g (47.83 mmol) of 4-(6-acryloyloxy-1-hexyloxy)phenol (manufactured by DKSH) represented by formula (IVa) was added thereto, and the reactor was immersed in an ice bath to allow the reaction The liquid internal temperature was 0°C. Next, 4.83 g (47.83 mmol) of triethylamine was slowly added dropwise over 5 minutes while keeping the internal temperature of the reaction liquid at 10° C. or lower. After completion of the dropwise addition, the entire contents were returned to 25° C. and stirred for further 1 hour.
[0293] 100 ml of distilled water was added to the obtained reaction liquid, and after washing|cleaning at 25 degreeC for 2 hours, the water layer was extracted. Further, the organic layer was washed twice with ...
Embodiment 2
[0297] (Example 2) Production of Mixture 2
[0298] The same operation as in Example 1 was performed except that 200 ml of CPME as a reaction solvent was replaced with 150 ml of tetrahydrofuran (THF). As a result, 17.91 g of a white solid was obtained.
[0299] The composition of the obtained white solid was confirmed by the same method as in Example 1. As a result, it was found that 12.89 g (30.81 mmol) of the target monoester, 4.98 g (7.49 mmol) of the diester, and 41 mg (0.24 mmol) of cyclohexanedicarboxylic acid. When the molar content was calculated from the respective composition ratios, the monoester content: 79.94 mol%, the diester content: 19.44 mol%, and the cyclohexanedicarboxylic acid content: 0.61 mol%.
Embodiment 3
[0300] (Example 3) Preparation of Mixture 3
[0301] The same operation as in Example 1 was performed except that 200 ml of CPME as a reaction solvent was replaced with 200 ml of methyl tert-butyl ether (MTBE). As a result, 17.70 g of a white solid was obtained.
[0302] The composition was confirmed by the same method, and it was found that 12.52 g (29.92 mmol) of the target monoester, 5.14 g (7.73 mmol) of the diester, and 43 mg (0.25 mmol) of cyclohexanedicarboxylic acid were included. . When the molar content was calculated from the respective composition ratios, the monoester content: 78.94 mol%, the diester content: 20.39 mol%, and the cyclohexanedicarboxylic acid content: 0.67 mol%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com