H3/H1N1(2009)/H1N1 influenza A virus multiplex fluorescence PCR detection kit and application thereof
A technology for detection kits and influenza viruses, which is applied in the field of multiple fluorescent PCR detection kits and virus detection kits. It can solve the problems that infections cannot be effectively distinguished, and consumes a lot of time and energy, and achieves short detection time and high accuracy. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Assembling of a multiplex fluorescence PCR detection kit for influenza A H3 / H1N1(2009) / H1N1 influenza virus nucleic acid
[0035] 1) Design primer / probe combination:
[0036] According to the nucleic acid sequence of each pathogen, sequence comparisons between the same species were performed to find conserved regions, and Primer Express 3.0 software was used to design primers and Taqman probes for multiple PCR on these conserved regions. Perform BLAST analysis on the NCBI website for each primer / probe combination obtained to ensure that it does not cross-react with other microorganisms that may be present in the sample. Finally, its performance is verified through experiments. The design of the internal control uses the same method. The designed primers / probes are shown in Table 1 below. The synthesis was completed by Shenggong Bioengineering (Shanghai) Co., Ltd.
[0037] Table 1 Primers and probes used to amplify A H3, new A H1N1 (2009), seasonal H1N1 influenza ...
Embodiment 2
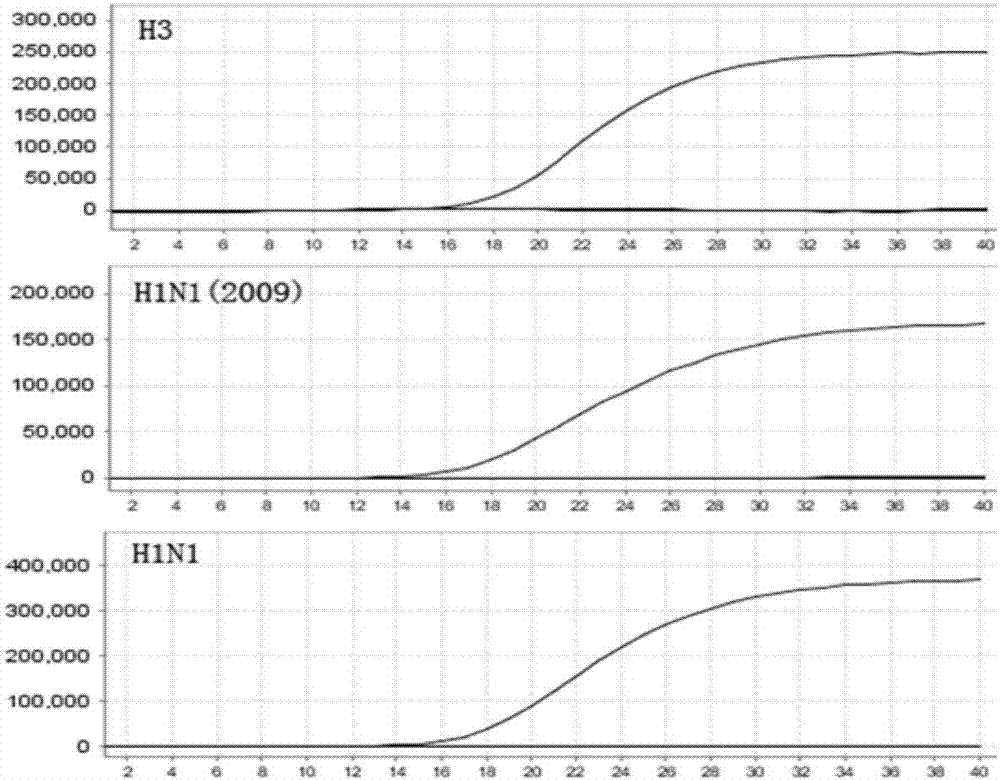
[0050] Example 2 Detection of influenza A H3, new A H1N1 (2009) and seasonal H1N1 influenza viruses respectively
[0051] The isolated and cultured strains of influenza A H3, new A H1N1 (2009) and seasonal H1N1 influenza viruses were used respectively, and the samples were tested with the kit prepared in Example 1.
[0052] The QIAamp MinElute Virus Spin Kit (QIAGEN) was used to extract nucleic acids from the samples. Then add H3 / H1N1(2009) / H1N1 primer probe mixture (2μL), enzyme system (2μL) and nucleic acid extracted from the sample or negative / positive control reagent (5μL) into 16μL of PCR reaction buffer. Subsequently, each reaction tube was subjected to PCR reaction on a suitable real-time fluorescent PCR machine (such as ABI 7500, Applied Biosystems) according to the following procedures: 50℃ for 25min, 95℃ for 5min; 95℃ for 10s, 55℃ for 15s, 72℃ Maintained for 30s, 5 cycles; 95°C for 10s, 60°C for 45s (collecting fluorescence signal), 40 cycles.
[0053] Result judgment:
[...
Embodiment 3
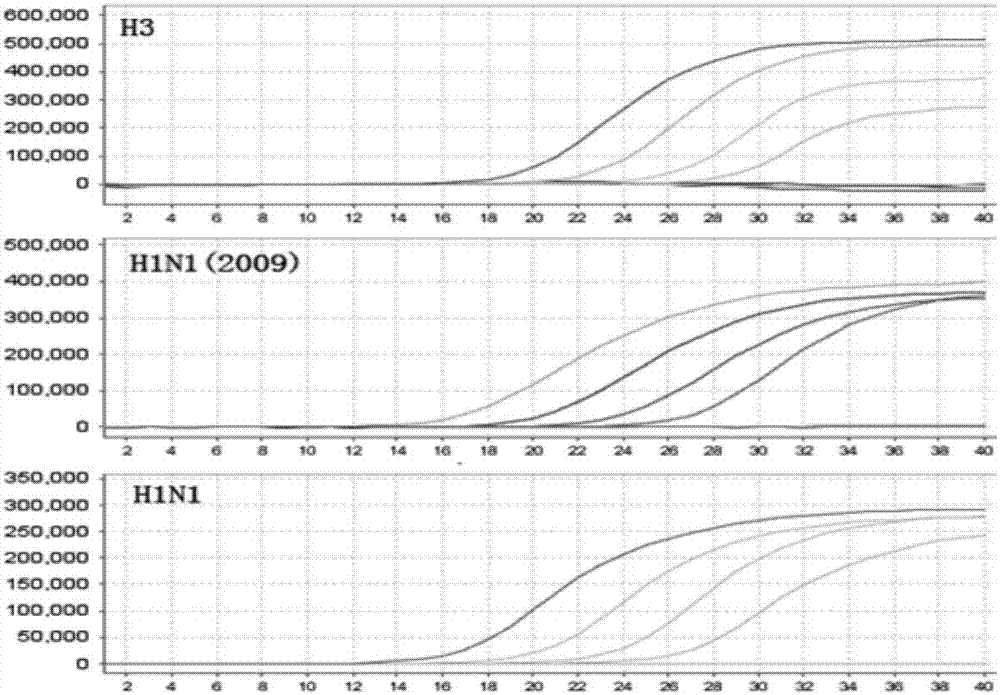
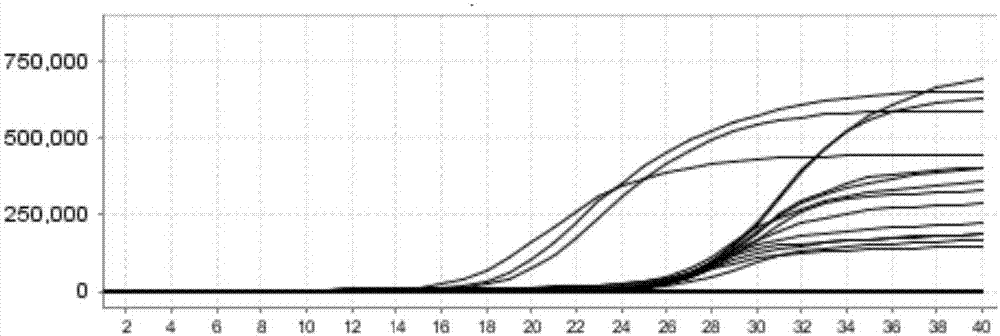
[0073] Example 3 One-time detection of influenza A H3, new A H1N1 (2009) and seasonal H1N1 influenza viruses
[0074] 1. Extraction of viral nucleic acid
[0075] The QIAamp MinElute Virus Spin Kit (QIAGEN) was used to extract viral nucleic acids from influenza A H3, new A H1N1 (2009) and seasonal H1N1 influenza virus samples. Perform a mixing operation on the extracted viral nucleic acid samples at the same ratio. Five gradients of 10-fold dilution are used as templates for detection.
[0076] 2. PCR amplification detection:
[0077] 1) Prepare PCR reaction solution according to the following composition (n is the number of reaction tubes).
[0078] The PCR reaction buffer is 16 μL×n, the H3 / H1N1(2009) / H1N1 primer probe mixture is 2 μL×n, and the enzyme system is 2 μL×n. (Note: Before use, ensure that the PCR reaction buffer, H3 / H1N1(2009) / H1N1 primer probe mixture is fully dissolved and mixed, and the enzyme system needs to be centrifuged before use to ensure that all enzymes are c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com