Application of a Streptomyces and its Metabolites Penicillin Compounds in Anti-kidney Cancer
A technique for tetromycin and streptomyces, which is applied in the directions of active ingredients of heterocyclic compounds, drug combinations, microorganism-based methods, etc., can solve the problems of weak mechanism research and the need for further exploration of anti-tumor potential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 Streptomyces sp.HBERC-58855 cultivation, identification, fermentation and HPLC analysis
[0034] 1. Solid culture of Streptomyces sp.HBERC-58855
[0035] Streptomyces sp.HBERC-58855 is isolated from the mangrove bottom mud. The strain is stored on the slope of ISP-2 medium. The composition of ISP-2 medium is: yeast extract powder 4g, glucose 4g, malt extract powder 10g, Coarse sea salt 30g, agar powder 20g, water 1000mL, pH 7.2~7.4.
[0036] 2. Identification of 16s rRNA gene of Streptomyces sp.HBERC-58855
[0037] Genomic DNA of Streptomyces sp.HBERC-58855 was extracted by microwave method and part of it was used as a template. Using 16S universal primers, rTaqase from Trans Gene Company was used to perform PCR amplification at 58°C as the annealing temperature and other conditions. The amplified PCR products were subjected to TA cloning and chemical transformation, and positive clones were sent for sequencing. Then the sequence obtained was spliced a...
Embodiment 2
[0043] Example 2 Separation, Purification and Structural Identification of Penicillins Compounds
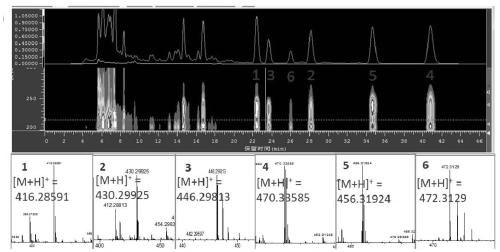
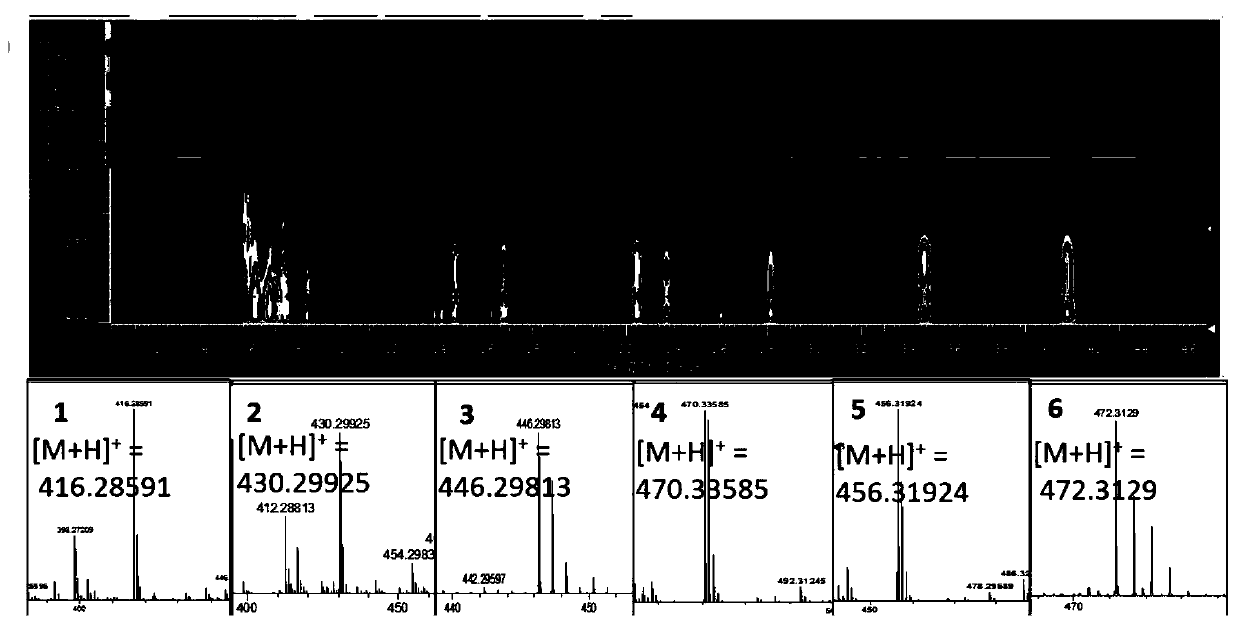
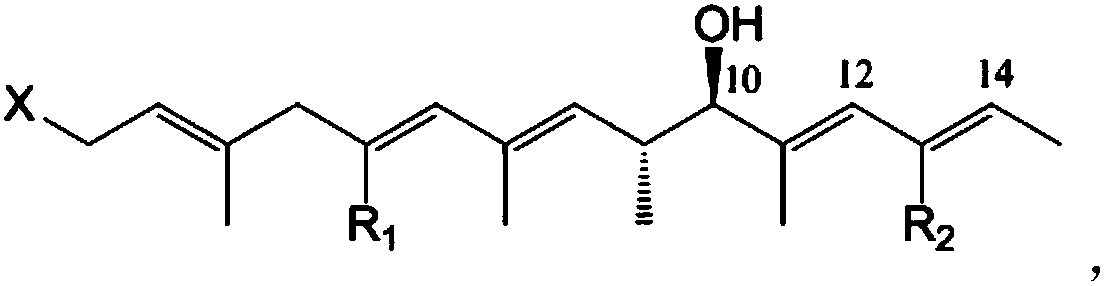
[0044] Streptomyces sp.HBERC-58855 strain fermented dry extract was dissolved in a small amount of methanol, mixed with silica gel, and subjected to medium pressure silica gel column chromatography (200-300 mesh), eluent was petroleum ether: dichloromethane: methanol system Gradient elution. 8 elution sites S1-S8 were obtained (petroleum ether; petroleum ether: dichloromethane 2:1; dichloromethane; dichloromethane: methanol 200:1; 100:1; 50:1; 30:1; methanol) . S2 was repeatedly separated and purified by preparative HPLC to obtain purified compounds 1 (Piericidin A, 242 mg), 4 (112 mg), 5 (67 mg) and 6 (14 mg). S3 was repeatedly separated and purified by preparative HPLC to obtain purified compounds 1 (82 mg), 2 (24 mg) and 3 (32 mg).
[0045] High resolution mass spectrometry HRMS and figure 1 consistent. The NMR H and C spectrum assignments of the six compounds are shown i...
Embodiment 3
[0053] Example 3 Inhibitory Activity of Penicillins Compounds to Three Lines of Renal Cancer Cells
[0054] Three human renal cell lines were ordered from Shanghai Cell Resource Center, Chinese Academy of Sciences: 786-O human renal cell line (Cat#TCHu186); ACHN human renal cell line (Cat#TCHu199); OS-RC-2 human renal cell line strain (Cat#TCHu40).
[0055] The inhibitory activity of renal cancer cells was tested by CCK-8 assay. Collect the cells in the logarithmic growth phase, count them, resuspend the cells with complete medium, adjust the cell concentration to an appropriate concentration (determined according to the results of the cell density optimization test), inoculate a 96-well plate, and add 100 μl of cell suspension to each well. Cells were incubated for 24 hours at 37°C, 100% relative humidity, 5% CO2 incubator. Dilute the compound to be tested to an appropriate concentration with medium, and add 25 μl / well to the cells. For ACHN cells, the final concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com