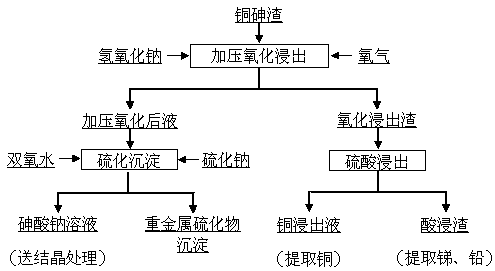
A method for separating copper and arsenic in copper-arsenic slag
A technology for arsenic residue and acid leaching residue, which is applied in the direction of improving process efficiency, can solve the problems of low arsenic leaching rate, difficult to achieve good separation of arsenic and copper, etc., and achieves the effect of low labor intensity, environmental friendliness, and efficient leaching.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Copper arsenic slag finely ground to a particle size of 100% less than 0.149mm, its main components are (%): Cu 41.48, As 22.85, Pb 3.09, Sb 2.68; industrial grade sodium hydroxide, wherein the content of sodium hydroxide is ≥96% ; Industrial grade sulfuric acid, where H 2 SO 4 Content ≥ 98%; industrial grade oxygen, of which O 2 Content ≥ 99%; industrial grade hydrogen peroxide, of which H 2 o 2 Content ≥ 27.5%; industrial grade sodium sulfide, of which Na 2 S content ≥ 60%.
[0019] Dissolve 72g of the above-mentioned industrial grade sodium hydroxide in 600ml of water and add it to a pressure reactor with a volume of 1L, then add 100g of copper arsenic slag, then slowly raise the temperature to 160°C, after the temperature is stabilized, introduce oxygen to keep the oxygen partial pressure at 0.20MPa , Control the stirring speed to 800 r / min and react for 6h. After the reaction is completed, turn off the oxygen and heat, pass cooling water into the reactor thro...
Embodiment 2
[0023] Copper arsenic slag finely ground to a particle size of 100% less than 0.149mm, its main components are (%): Cu 50.65, As 25.28, Pb 4.86, Sb 5.64; industrial grade sodium hydroxide, wherein the content of sodium hydroxide is ≥96% ; Industrial grade sulfuric acid, where H 2 SO 4 Content ≥ 98%; industrial grade oxygen, of which O 2 Content ≥ 99%; industrial grade hydrogen peroxide, of which H 2 o 2 Content ≥ 27.5%; industrial grade sodium sulfide, of which Na 2 S content ≥ 60%.
[0024] Dissolve 96g of the above-mentioned industrial grade sodium hydroxide in 800ml of water and add it to a pressure reactor with a volume of 1L, then add the above-mentioned 100g of copper arsenic slag, control the stirring speed at 800r / min, then slowly raise the temperature to 220°C, and pass the Oxygen was introduced to keep the oxygen partial pressure at 0.50MPa and react for 2h. After the reaction was completed, cooling water was passed into the reaction kettle through the cooling ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com