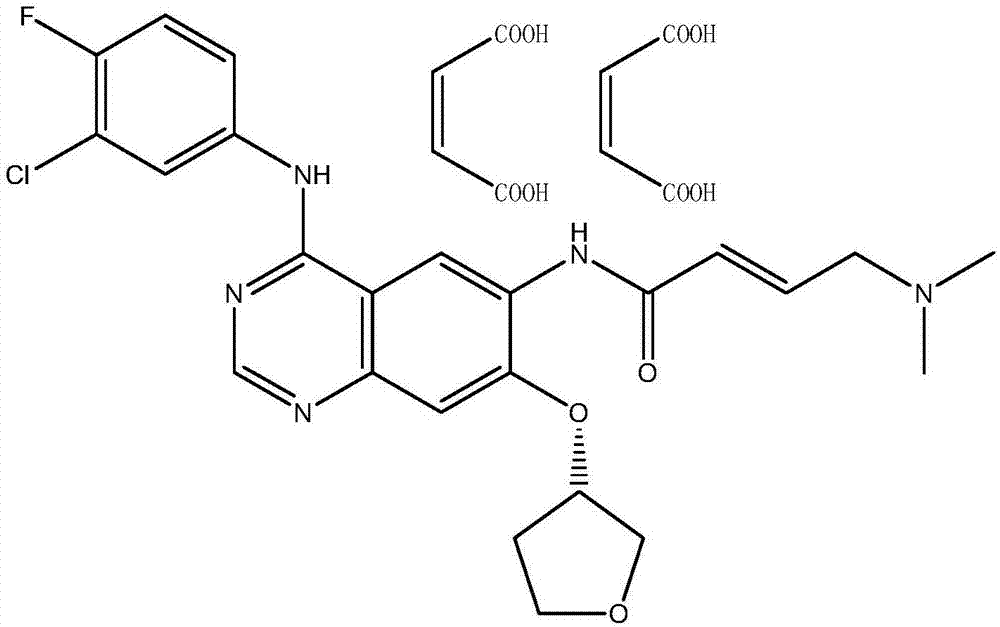
Preparation method for afatinib
A technology of afatinib and reaction, which is applied in the field of afatinib preparation, can solve the problems of large loss, difficult removal, and many impurities, and achieve the effect of low cost, less impurities, and reduced loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
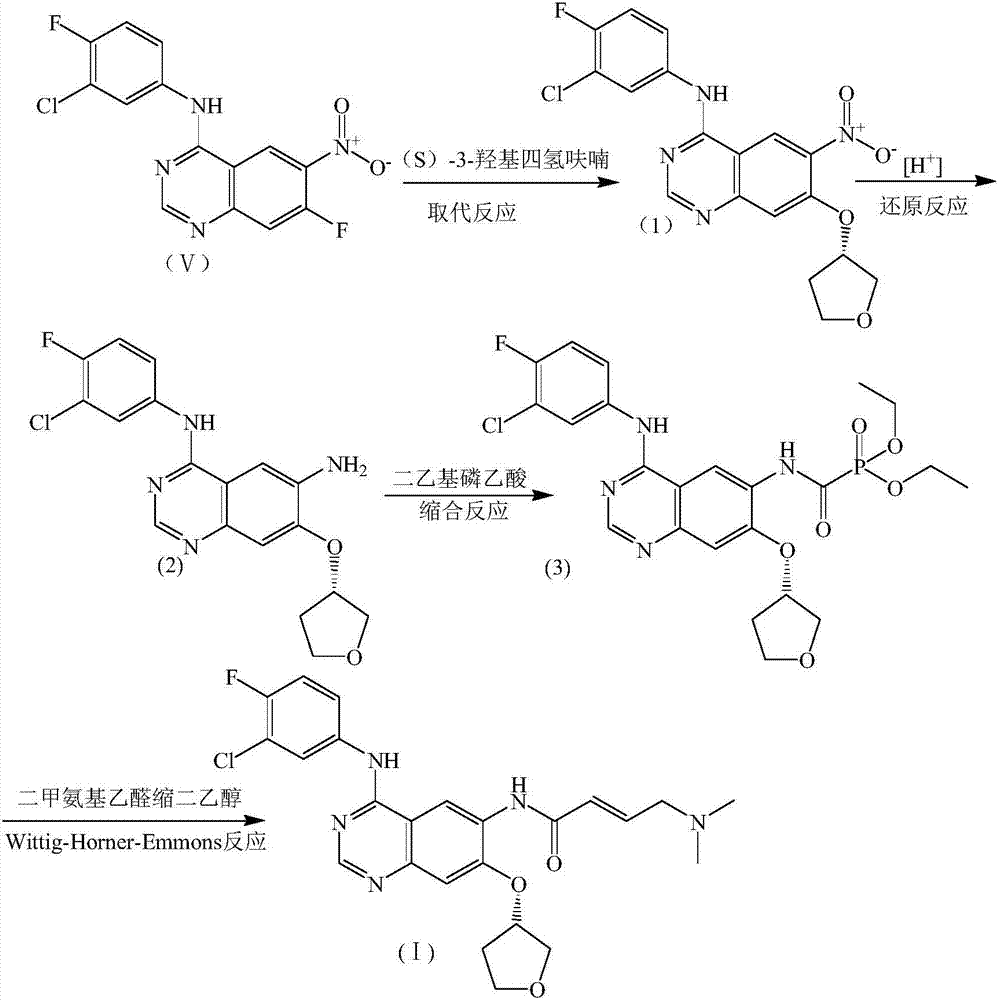
[0038] first step:
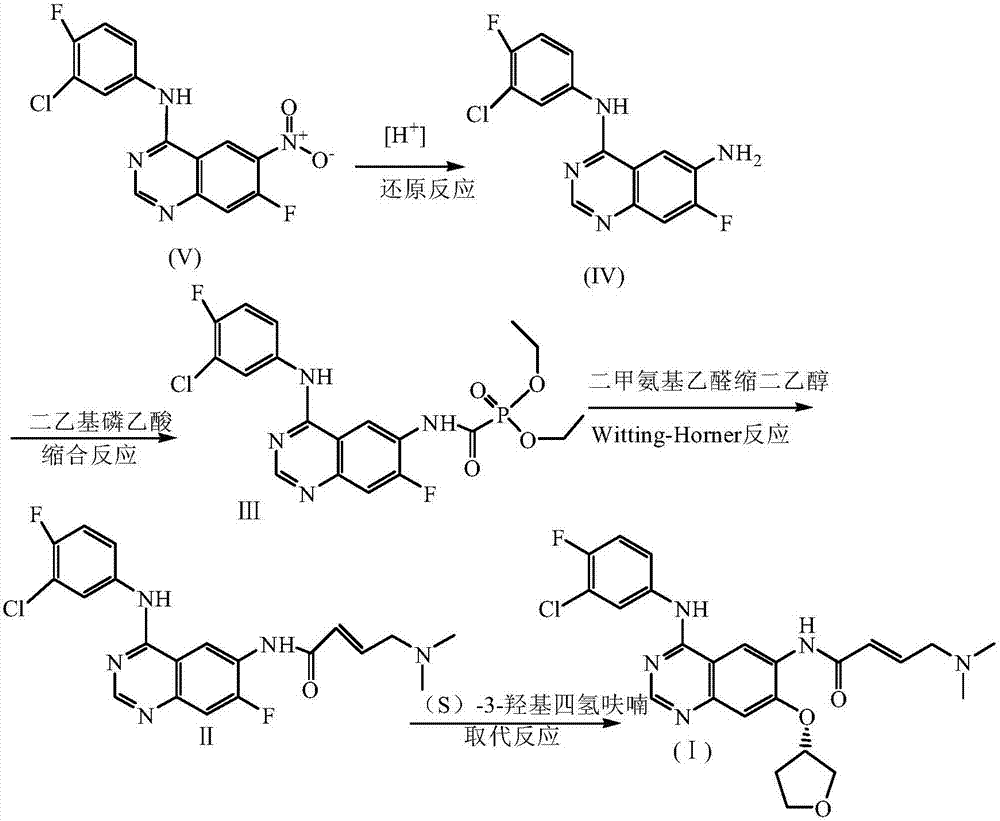
[0039] Take 3.4g compound V, 153mg FeCl 3 , 50mL of a mixed solution of tert-butanol and acetone (the volume ratio of tert-butanol: acetone is 3:4) is put into a single-necked flask, electromagnetic stirring is started, the air is replaced by nitrogen 3 times, and the nitrogen is replaced by hydrogen 3 times, and the temperature and pressure are maintained for 4 hours. After completion, the catalyst was removed by suction filtration under reduced pressure, and the filtrate was rotary evaporated to dryness and dried to obtain intermediate IV with a yield of 99.6%.
[0040] The second step:
[0041] Take 2.0 g of diethyl phosphoacetic acid and 50 ml of tetrahydrofuran into a 250 ml three-necked flask, start stirring, slowly add 2.0 g of CDI, keep the reaction at 40°C for 1 hour, then add 3.0 g of Intermediate IV, continue the reaction for 1 hour, after the reaction is complete, a large amount of solids are deposited , Then add 50ml methyl tert-butyl ether, stir fo...
Embodiment 2
[0048] first step:
[0049] Take 3.4g of compound V, 102mg of 10% Pd / C, and 50mL of methyl tert-butyl ether into a single-necked flask, start electromagnetic stirring, replace air with nitrogen 3 times, replace nitrogen with hydrogen 3 times, keep the temperature and keep the pressure for 4 hours, the reaction is complete, reduce The catalyst was removed by suction filtration, the filtrate was evaporated to dryness and dried to obtain Intermediate IV with a yield of 99.1%.
[0050] The second step:
[0051] Take 2.0g of diethyl phosphoacetic acid and 50ml of methyl tert-butyl ether into a 250ml three-necked flask, start stirring, slowly add 4.5g of CDI, keep the reaction at 40°C for 1 hour, then add 3.0g of Intermediate IV, and continue the reaction for 1 hour. After completion, a large amount of solids were precipitated, and then 50 ml of methyl tert-butyl ether was added, and after stirring for 1 h, the intermediate III was obtained by suction filtration and drying. The yield was ...
Embodiment 3
[0058] first step:
[0059] Take 3.4g compound V, 170mg Fe(OH) 3 , 100mL acetone into a single-neck flask, start electromagnetic stirring, replace the air with nitrogen 3 times, replace the nitrogen with hydrogen 3 times, heat preservation and pressure reaction for 6h, after the reaction is completed, the catalyst is removed by vacuum filtration, the filtrate is evaporated to dryness, and the intermediate is dried. Ⅳ, the yield is 99.0%.
[0060] The second step:
[0061] Take 2.0g of diethyl phosphoacetic acid and 80ml of methanol into a 250ml three-necked flask, start stirring, slowly add 0.9g of DCC, keep the reaction at 40℃ for 1h, then add 3.0g of Intermediate IV, continue to keep the temperature for 1h, the reaction is complete, a large amount of precipitation For the solid, add 50ml of methanol, stir for 1h, filter with suction and dry to obtain Intermediate III with a yield of 96.2%.
[0062] third step:
[0063] Firstly, dimethylaminoacetaldehyde diethanol is deprotected to p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com