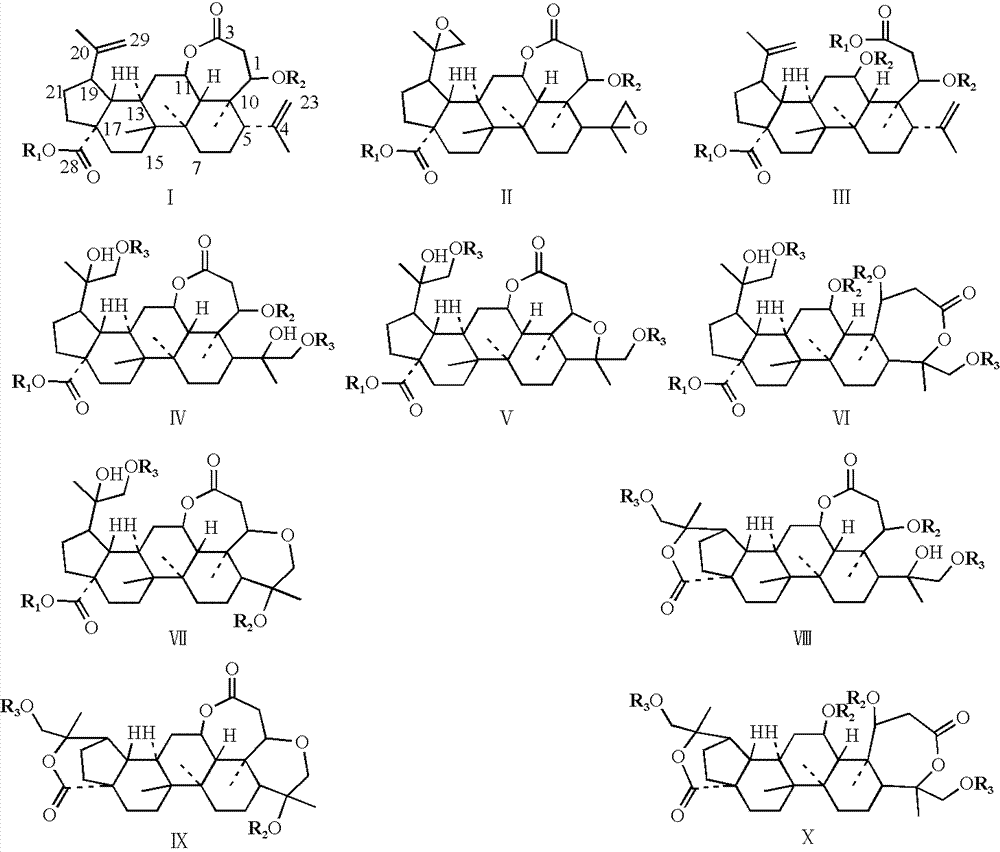
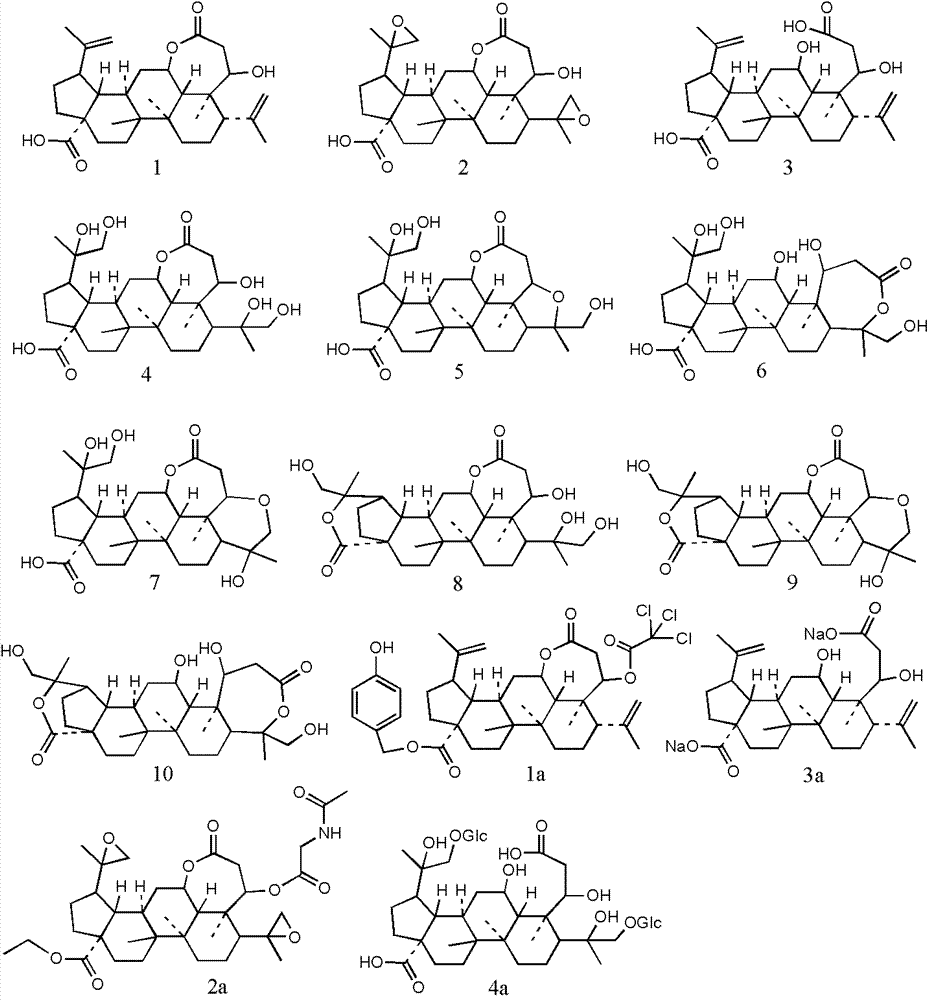
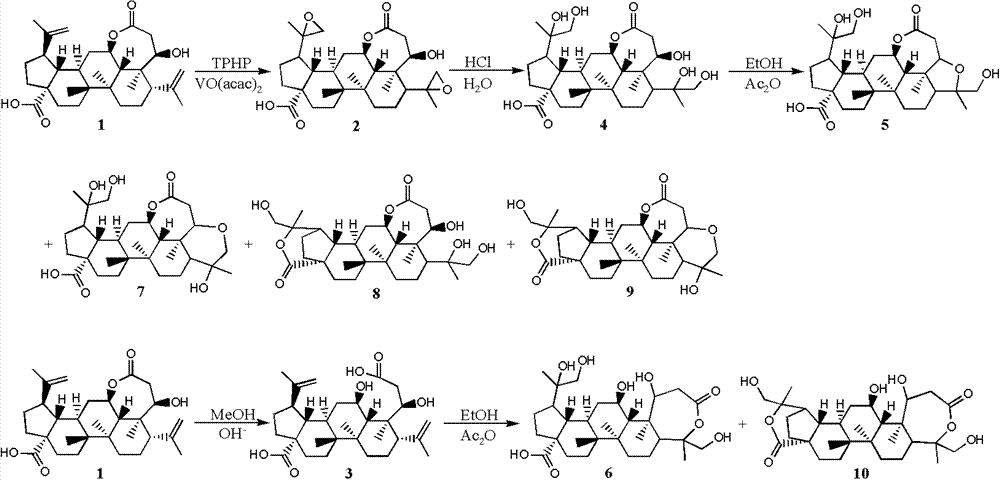
Split-ring lupinane derivatives and medicinal application thereof
A technology of lupinane and derivatives is applied in the field of split-ring lupinane derivatives and pharmaceutical compositions containing them, and can solve the problems of shortening sleep latency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Synthesis of split ring lupin derivatives
[0029] 1.1 Instruments and reagents
[0030] 1 HNMR nuclear magnetic resonance spectrum adopts Bruker AVII 500 superconducting nuclear magnetic resonance instrument; HRMS mass spectrometry adopts UPLC-XevoG 2 -S QTOF-MS / MS. Derivative 1 is self-made in the laboratory (purity ≥ 98%), and other reagents are domestic analytical reagents.
[0031] 1.2 Synthesis of derivatives
[0032] 1.2.1 Synthesis of Derivative 2
[0033] Dissolve derivative 1 (484.0mg, 1.0mmol) in 50mL of dichloromethane, add vanadyl acetylacetonate (26.0mg, 0.1mmol), stir well and add 70% tert-butyl hydroperoxide aqueous solution (413μL, 3mmol) , react at room temperature for 3 hours, add 30mL aqueous sodium bicarbonate solution, stir for 15 minutes, extract 3 times with ethyl acetate 30mL, combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, and recover the solvent under reduced pressure to obtain derivati...
Embodiment 2
[0065] Example 2: Test of melatonin receptor agonistic activity of split-lupin derivatives
[0066] 2.1 Method: Homogeneous Time-Resolved Fluorescence Method (Zhang Xuan. New synthesis method and structural transformation of melatonin receptor agonist ramelteon and design and synthesis of single-molecule multi-target anti-tumor drugs[D]. East China Normal University University, 2014.).
[0067] 2.2 Results: According to the results obtained by the above method, the prepared split-lupin derivatives 1-10, 1a-4a showed certain activities against MT1 and MT2, as shown in Table 1.
[0068] Table 1 EC of split ring lupin derivatives 50 Value (nM)
[0069]
[0070] 2.3 Conclusion: The prepared split ring lupine derivatives 1-10, 1a-4a have certain activities on MT1 and MT2, and have the potential to prevent, slow down or treat diseases related to central nervous system dysfunction. Potential drug development value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com