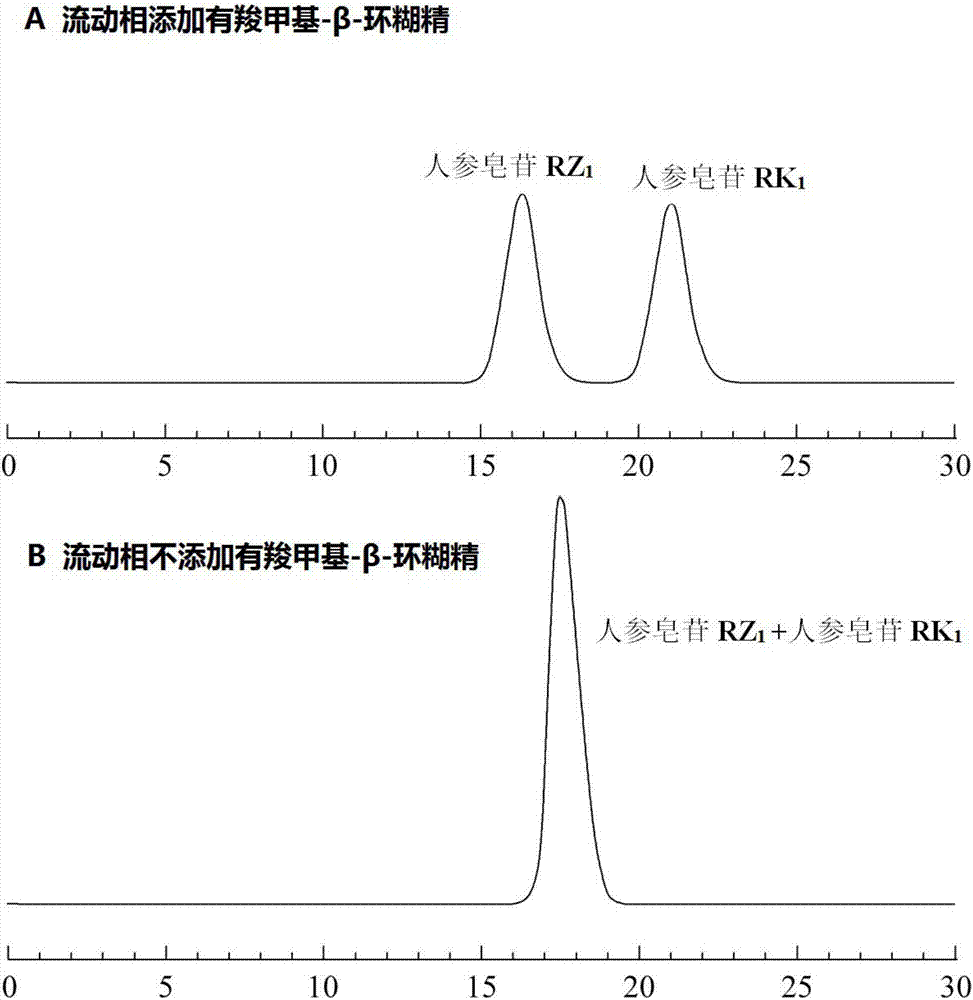
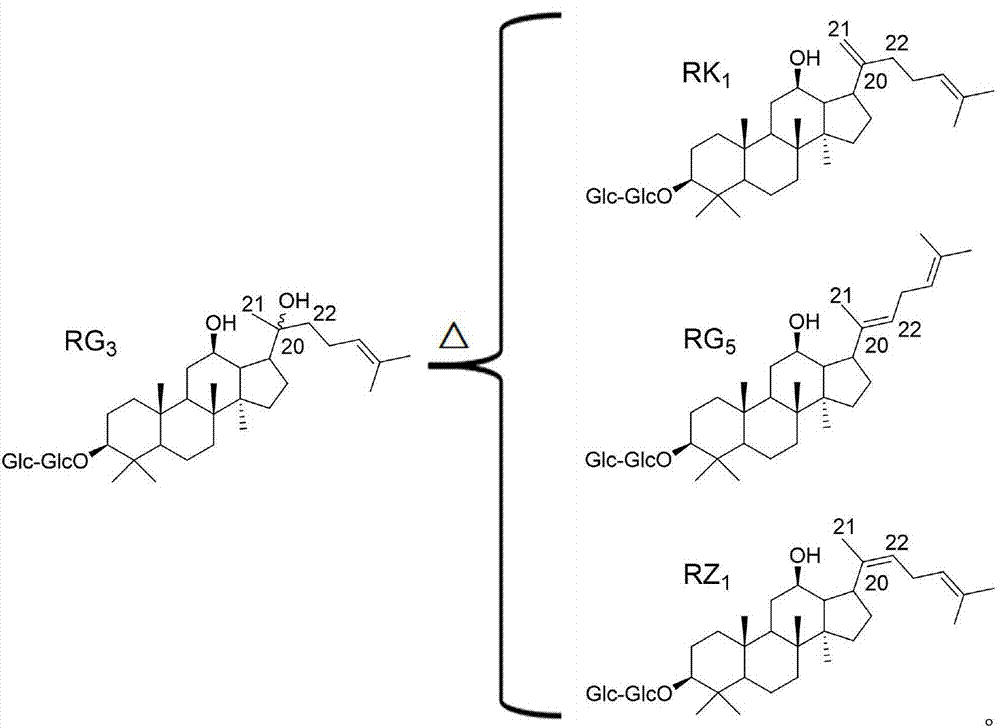
Method for measuring isomer impurity ginsenoside RK1 in ginsenoside RZ1 raw material or preparation
A technology of ginsenosides and isomers, applied in the field of drug analysis, can solve the problems of poor reproducibility, inability to meet drugs, immature development of two-dimensional liquid chromatography, etc., and achieve the effect of high resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: mobile phase is aqueous methanol
[0029] 1. Test material
[0030] Waters2695 liquid chromatograph (Waters, USA);
[0031] Sartorius electronic balance (Germany Sartorius);
[0032] Column: Waters XBridge TM C18 chromatographic column (5μm, 4.6×250mm; Waters, USA);
[0033] Ginsenoside RZ 1 and ginsenoside RK 1 The standard products are self-made, and the purity is greater than 98%;
[0034] Methanol, 85% phosphoric acid and triethylamine are chromatographically pure, water is deionized water, and carboxymethyl-β-cyclodextrin is analytically pure.
[0035] 2. Test method and test results
[0036] 2.1 Solution preparation
[0037] Chromatographic peak positioning solution: prepared containing ginsenoside RZ 1 or ginsenoside RK 1 single standard solution, concentration 0.1mg / mL.
[0038] System Suitability Solution: Formulating Ginsenoside RZ 1 and ginsenoside RK 1 The mixed standard solution with a concentration of 0.5 mg / mL was used as the...
Embodiment 2
[0061] Embodiment 2: mobile phase is acetonitrile aqueous solution
[0062] 1. Test material
[0063] Waters2695 liquid chromatograph (Waters, USA);
[0064] Sartorius electronic balance (Germany Sartorius);
[0065] Column: Waters XBridge TM C18 chromatographic column (5μm, 4.6×250mm; Waters, USA);
[0066] Ginsenoside RZ 1 and ginsenoside RK 1 The standard products are self-made, and the purity is greater than 98%;
[0067] Acetonitrile, 85% phosphoric acid, and triethylamine are chromatographically pure, water is deionized water, and carboxymethyl-β-cyclodextrin is analytically pure.
[0068] 2. Test method and test result
[0069] 2.1 Solution preparation
[0070] Chromatographic peak positioning solution: prepared containing ginsenoside RZ 1 or ginsenoside RK 1 single standard solution, concentration 0.1mg / mL.
[0071] System Suitability Solution: Formulating Ginsenoside RZ 1 and ginsenoside RK 1 The mixed standard solution with a concentration of 0.5 mg / m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com