Skin medicine composition with azulene sulfonate
A technology of a composition and azulene sulfonic acid, which can be used in drug combinations, medical preparations containing active ingredients, skin diseases, etc., and can solve problems such as pigmentation, telangiectasia, and skin atrophy and thinning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1 suspension
[0051] Active ingredient list
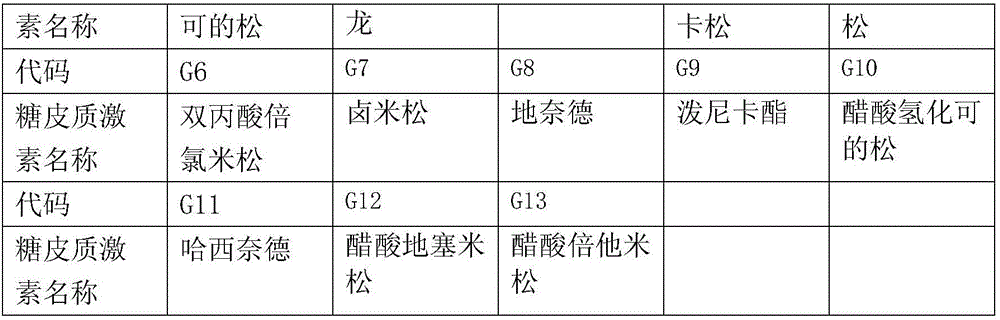
[0052]
[0053] Adjuvant prescription and preparation method of embodiment 1
[0054] Appropriate amount of sodium chloride, sodium carboxymethylcellulose 2.5g, poloxamer 0.5g, add distilled water to 1000g
[0055] Preparation method: dissolve the carboxymethylcellulose sodium and poloxamer of the prescribed amount in 500ml water for injection, stir to dissolve, add the azulene sulfonic acid compound of the prescribed amount; then add the glucocorticoid drugs of the prescribed amount, Add the remaining amount of water for injection. Adjust to isotonicity with sodium chloride and dispense.
Embodiment 1
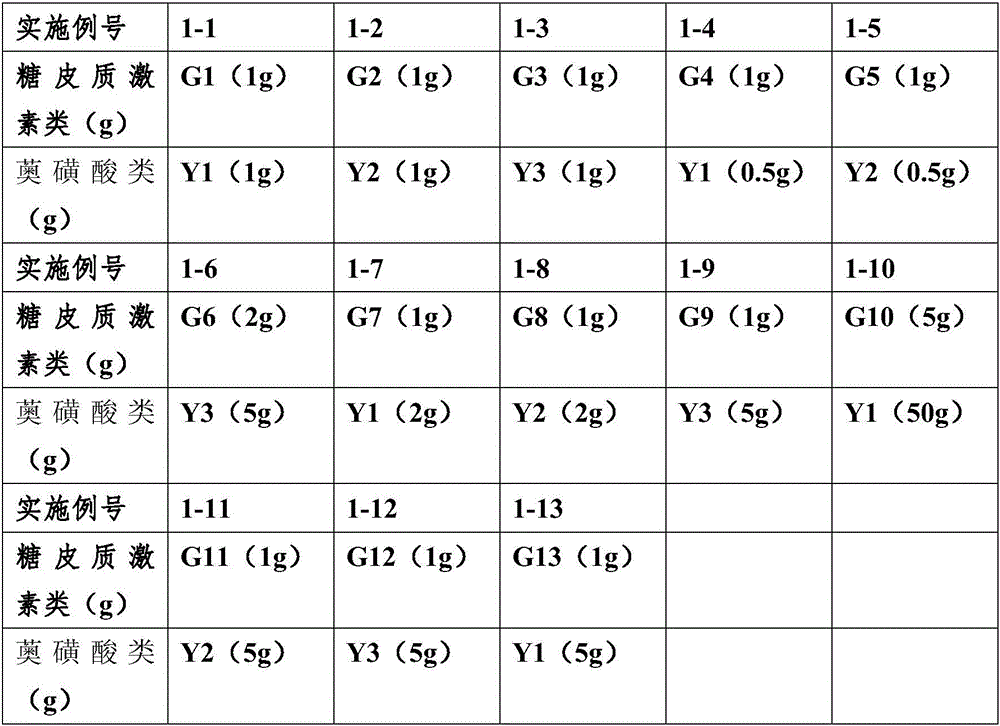
[0057] According to the adjuvant prescription and preparation method in Example 1, prepare azulene sulfonic acid compound suspension and glucocorticoid drug suspension respectively, the number of comparative example 1 corresponds to the number of Example 1, according to the activity used The components are different, and the end number of the comparative examples is increased by G (glucocorticoid drugs) or Y (azulene sulfonic acid compounds). For example, the active ingredients of Example 1-1 are G1 (1g) and Y1 (1g), while the active ingredients of Comparative Example 1-1-G are glucocorticoids (no azulene sulfonic acid drugs), and the active ingredients are G1 (1 g), the active ingredient of the suspension 1-1-Y of the comparative example is azulene sulfonic acid drugs (no glucocorticoid drugs), and the active ingredient is Y1 (1 g).
Embodiment 2
[0058] Embodiment 2 gel
[0059] Active ingredient list
[0060]
[0061] Adjuvant prescription and preparation method of embodiment 2
[0062] See the above table for the dosage of active ingredients glucocorticoids and azulene sulfonic acids
[0063] Polyethylene glycol 600-100g, propylene glycol 100g, absolute ethanol 100g, carbomer 93430g, appropriate amount of triethanolamine, add distilled water to 1000g.
[0064] Preparation method: Sprinkle Carbomer 934 evenly on the surface of 300ml distilled water, let it stand still to make it fully swell; mix the prescribed amount of glucocorticoids and azulene sulfonic acid drugs with absolute ethanol, and then add Prescription measures of water, macrogol 600, and propylene glycol. Mix and stir the above components, slowly add triethanolamine dropwise, stir while adding, control pH 6.0-7.0, and then pack it separately.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com