A high thermal conductivity binary low eutectic hydrated salt phase change material and its preparation method
A phase change material and low eutectic technology, applied in the field of material chemistry, can solve the problems of cumbersome preparation process, high degree of supercooling, and complicated operation of phase change materials, and achieve improved thermal cycle stability, low price, and broad application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
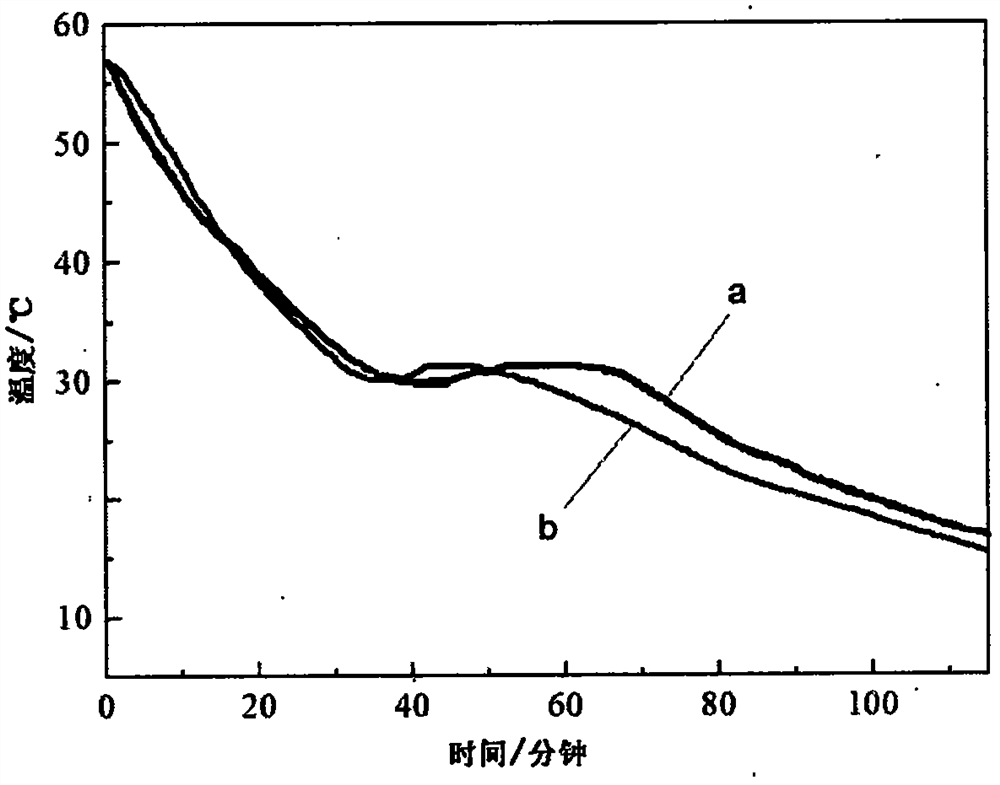
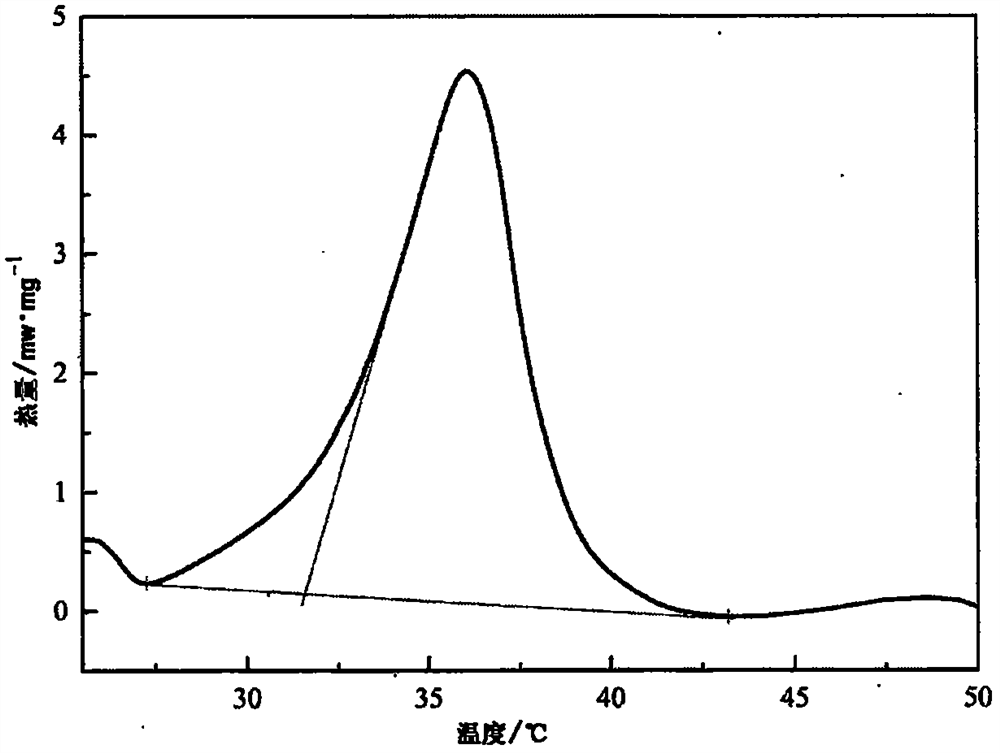
Image
Examples
specific Embodiment approach 1
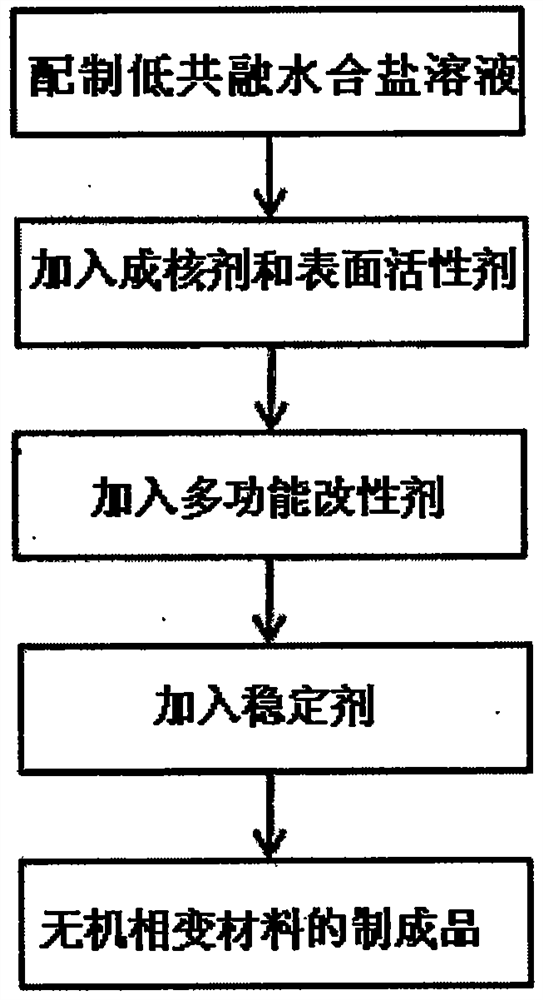
[0027] Specific implementation mode one: combine figure 1 Describe this embodiment, in this embodiment, the high thermal conductivity binary low eutectic hydrated salt phase change material is composed of 23-26 parts by weight of Glauber's salt, 70-75 parts of disodium hydrogen phosphate dodecahydrate, 0.5- A solution composed of 1.5 parts of nucleating agent, 1-2.25 parts of surfactant, 3-4.5 parts of multifunctional modifier and 1-2.25 parts of stabilizer.
[0028] Glauber's salt is Na in the present invention 2 SO 4 10H 2 O, disodium hydrogen phosphate dodecahydrate is Na 2 HPO 4 12H 2 O.
specific Embodiment approach 2
[0029] Embodiment 2: This embodiment is a further limitation of Embodiment 1. In this embodiment, the weight-number ratio between Glauber's salt and disodium hydrogen phosphate dodecahydrate is 1:3. This ratio is the best ratio in parts by weight between Glauber's salt and disodium hydrogen phosphate dodecahydrate obtained through multiple sample tests.
specific Embodiment approach 3
[0030] Specific implementation mode three: combination figure 1 Describe this embodiment mode, this preparation method is carried out according to the following steps in this embodiment mode:
[0031] Step 1: Weigh 23-26 parts of Glauber's salt, 70-75 parts of disodium hydrogen phosphate dodecahydrate, 0.5-1.5 parts of nucleating agent, 1-2.25 parts of surfactant, 3- 4.5 parts of multifunctional modifier and 1-2.25 parts of stabilizer;
[0032] Step 2: Pour 23-26 parts of Glauber's salt and 70-75 parts of disodium hydrogen phosphate dodecahydrate into a container and mix, heat at 50-70°C until completely melted, then stir evenly to prepare a low eutectic hydration saline solution;
[0033] Step 3: Under the condition of stirring the eutectic hydrated salt solution, add 0.5-1.5 parts of nucleating agent and 1-2.25 parts of surfactant in sequence until the two are completely dissolved to form a preliminary modified solution;
[0034] Step 4: Add 3 to 4.5 parts of a multifunct...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com