Monoclonal antibody capable of secreting anti-streptococcus suis 2 AK (Aspartokinase) protein and application thereof
A monoclonal antibody, Streptococcus suis technology, applied in the field of immunological detection of pathogenic microorganisms, to achieve the effect of high antibody titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Immunization and cell fusion, screening and cloning
[0029] 1. Use 6-week-old female Balb / c mice. Equal volumes of Freund's complete adjuvant and recombinant AK protein ("Prokaryotic expression and antigenicity detection of aspartokinase of Streptococcus suis type 2" Chinese Journal of Etiology, 2017, 12 (4)) were emulsified in equal volumes as immunogens. The amount of antigen immunization was injected subcutaneously in each mouse with a dose of 100 μg; additional immunization was given every 14 days, and emulsified with Freund’s incomplete adjuvant for a total of 3 times, and the amount of immunogen injection remained unchanged; 3 days before cell fusion, intraperitoneal injection For immunization, the immunization dose is still 100 μg / mouse.
[0030] 2. Cell fusion, screening and cloning
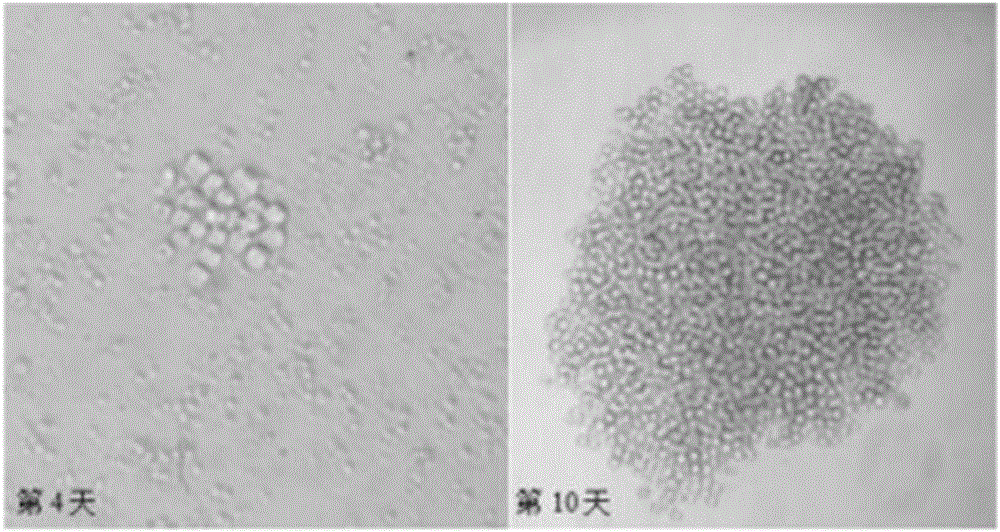
[0031] Spleen cells (1×10 7 ) and SP2 / 0 cells (2×10 6 ), then add 50% PEG1459 preheated to 37°C for fusion, the fused cells are suspended in HAT medium, and distrib...
Embodiment 2
[0032] Embodiment 2: Indirect ELASA method detects antibody
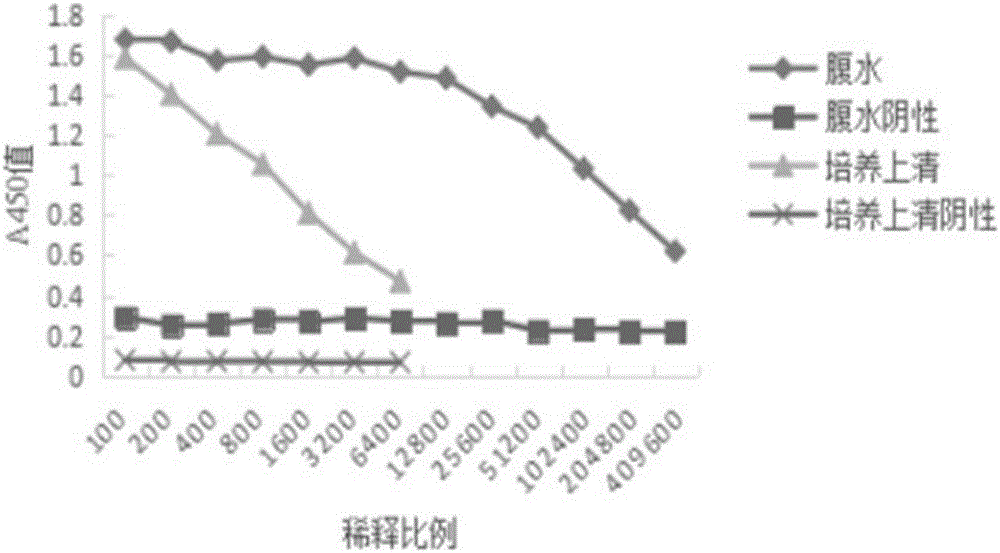
[0033] After screening and serial cloning, a hybridoma cell line stably secreting anti-AK monoclonal antibody was obtained, which was named 2E9. Inject the hybridoma cell 2E9 into the peritoneal cavity of the mouse (10 days before intraperitoneal injection of paraffin), 2×10 5 Ascites was collected after the abdominal distension of the mice. Antibodies were detected by indirect ELASA method.
[0034]Add 100 μl of 5 μg / ml recombinant AK protein to each well, coat overnight at 4°C; wash the plate three times with PBST, add 300 μl of 3% BSA blocking solution, and block for 2 hours at 37°C; discard the blocking solution, wash the plate three times with PBST, add 100 μl of hybridoma cells for culture Supernatant, incubated at 37°C for 1 hour; washed three times with PBST, added enzyme-labeled secondary antibody, incubated at 37°C for 1 hour; washed three times with PBST, added TMB for color development.
[0035] The r...
Embodiment 3
[0036] Embodiment 3: Western blot identification anti-AK monoclonal antibody
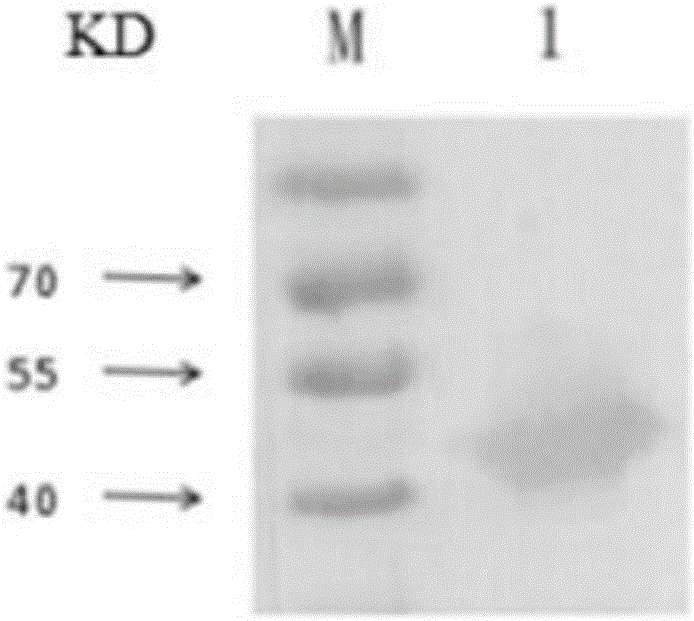
[0037] The recombinant AK protein was subjected to 10% SDS-PAGE, and then transferred to PVDF membrane after electrophoresis; 5% skimmed milk powder was blocked at 37°C for 1 hour, and washed three times with TBST; 2E9 ascites (1:5000) was incubated, overnight at 4°C, and washed three times with TBST; HRP- Incubate with goat anti-mouse (1:5000), at 37°C for 1 hour, wash three times with TBST; the bands are displayed by DAB color development.
[0038] Western blot results ( image 3 ) shows that there is a reactive band at 50KD between 2E9 ascites and recombinant AK protein. Monoclonal antibody can specifically react with AK protein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com